In vitro maturation of NiSOD reveals a role for cytoplasmic histidine in processing and metalation
- PMID: 37723610
- PMCID: PMC10628968
- DOI: 10.1093/mtomcs/mfad054
In vitro maturation of NiSOD reveals a role for cytoplasmic histidine in processing and metalation
Abstract
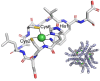
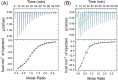
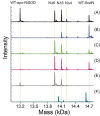
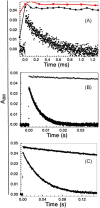
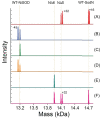
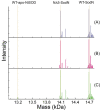
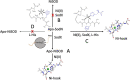
The importance of cellular low molecular weight ligands in metalloenzyme maturation is largely unexplored. Maturation of NiSOD requires post-translational N-terminal processing of the proenzyme, SodN, by its cognate protease, SodX. Here we provide evidence for the participation of L-histidine in the protease-dependent maturation of nickel-dependent superoxide dismutase (NiSOD) from Streptomyces coelicolor. In vitro studies using purified proteins cloned from S. coelicolor and overexpressed in E. coli support a model where a ternary complex formed between the substrate (SodN), the protease (SodX) and L-Histidine creates a novel Ni-binding site that is capable of the N-terminal processing of SodN and specifically incorporates Ni into the apo-NiSOD product. Thus, L-Histidine serves many of the functions associated with a metallochaperone or, conversely, eliminates the need for a metallochaperone in NiSOD maturation.
Keywords: in vitro maturation; low-molecular weight ligands; nickel; post-translational modification; protease (SodX); superoxide dismutase.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
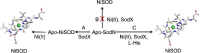


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

