CSNK1A1/CK1α suppresses autoimmunity by restraining the CGAS-STING1 signaling
- PMID: 37723657
- PMCID: PMC10813568
- DOI: 10.1080/15548627.2023.2256135
CSNK1A1/CK1α suppresses autoimmunity by restraining the CGAS-STING1 signaling
Abstract
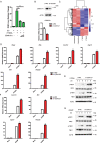
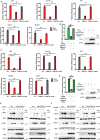
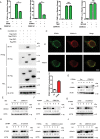
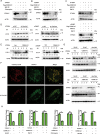
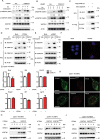
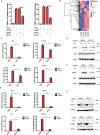
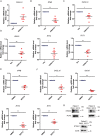
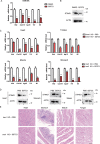
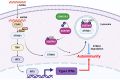
STING1 (stimulator of interferon response cGAMP interactor 1) is the quintessential protein in the CGAS-STING1 signaling pathway, crucial for the induction of type I IFN (interferon) production and eliciting innate immunity. Nevertheless, the overactivation or sustained activation of STING1 has been closely associated with the onset of autoimmune disorders. Notably, the majority of these disorders manifest as an upregulated expression of type I interferons and IFN-stimulated genes (ISGs). Hence, strict regulation of STING1 activity is paramount to preserve immune homeostasis. Here, we reported that CSNK1A1/CK1α, a serine/threonine protein kinase, was essential to prevent the overactivation of STING1-mediated type I IFN signaling through autophagic degradation of STING1. Mechanistically, CSNK1A1 interacted with STING1 upon the CGAS-STING1 pathway activation and promoted STING1 autophagic degradation by enhancing the phosphorylation of SQSTM1/p62 at serine 351 (serine 349 in human), which was critical for SQSTM1-mediated STING1 autophagic degradation. Consistently, SSTC3, a selective CSNK1A1 agonist, significantly attenuated the response of the CGAS-STING1 signaling by promoting STING1 autophagic degradation. Importantly, pharmacological activation of CSNK1A1 using SSTC3 markedly repressed the systemic autoinflammatory responses in the trex1-/- mouse autoimmune disease model and effectively suppressed the production of IFNs and ISGs in the PBMCs of SLE patients. Taken together, our study reveals a novel regulatory role of CSNK1A1 in the autophagic degradation of STING1 to maintain immune homeostasis. Manipulating CSNK1A1 through SSTC3 might be a potential therapeutic strategy for alleviating STING1-mediated aberrant type I IFNs in autoimmune diseases.Abbreviations: BMDMs: bone marrow-derived macrophages; cGAMP: cyclic GMP-AMP; CGAS: cyclic GMP-AMP synthase; HTDNA: herring testes DNA; IFIT1: interferon induced protein with tetratricopeptide repeats 1; IFNA4: interferon alpha 4; IFNB: interferon beta; IRF3: interferon regulatory factor 3; ISD: interferon stimulatory DNA; ISGs: IFN-stimulated genes; MEFs: mouse embryonic fibroblasts; PBMCs: peripheral blood mononuclear cells; RSAD2: radical S-adenosyl methionine domain containing 2; SLE: systemic lupus erythematosus; STING1: stimulator of interferon response cGAMP interactor 1; TBK1: TANK binding kinase 1.
Keywords: Autoimmunity; CSNK1A1; STING1; autophagy; type I IFN.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
Similar articles
-
SESN1 negatively regulates STING1 to maintain innate immune homeostasis.Autophagy. 2025 Jun;21(6):1245-1262. doi: 10.1080/15548627.2025.2463148. Epub 2025 Feb 13. Autophagy. 2025. PMID: 39945079
-
UXT attenuates the CGAS-STING1 signaling by targeting STING1 for autophagic degradation.Autophagy. 2023 Feb;19(2):440-456. doi: 10.1080/15548627.2022.2076192. Epub 2022 May 30. Autophagy. 2023. PMID: 35543189 Free PMC article.
-
Cordycepin ameliorates autoimmunity by promoting STING degradation via autophagy pathway.Br J Pharmacol. 2025 Apr;182(7):1546-1560. doi: 10.1111/bph.17425. Epub 2024 Dec 15. Br J Pharmacol. 2025. PMID: 39675775
-
The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy.Acta Pharm Sin B. 2022 Jan;12(1):50-75. doi: 10.1016/j.apsb.2021.05.011. Epub 2021 May 20. Acta Pharm Sin B. 2022. PMID: 35127372 Free PMC article. Review.
-
The interferon response to intracellular DNA: why so many receptors?Immunobiology. 2013 Nov;218(11):1312-21. doi: 10.1016/j.imbio.2013.07.007. Epub 2013 Jul 29. Immunobiology. 2013. PMID: 23962476 Review.
Cited by
-
SESN1 negatively regulates STING1 to maintain innate immune homeostasis.Autophagy. 2025 Jun;21(6):1245-1262. doi: 10.1080/15548627.2025.2463148. Epub 2025 Feb 13. Autophagy. 2025. PMID: 39945079
-
Macrophages Unmasked: Their Pivotal Role in Driving Atherosclerosis in Systemic Lupus Erythematosus.Clin Rev Allergy Immunol. 2025 Feb 7;68(1):10. doi: 10.1007/s12016-025-09025-6. Clin Rev Allergy Immunol. 2025. PMID: 39920534 Review.
-
STING exerts antiviral innate immune response by activating pentose phosphate pathway.Cell Commun Signal. 2024 Dec 18;22(1):599. doi: 10.1186/s12964-024-01983-2. Cell Commun Signal. 2024. PMID: 39695767 Free PMC article.
-
The cGAS-STING/PERK-eIF2α: Individual or Potentially Collaborative Signaling Transduction in Cardiovascular Diseases.Int J Biol Sci. 2024 Oct 28;20(15):5868-5887. doi: 10.7150/ijbs.101247. eCollection 2024. Int J Biol Sci. 2024. PMID: 39664570 Free PMC article. Review.
-
VRK1 promotes DNA-induced type I interferon production.Mol Biol Rep. 2024 Mar 27;51(1):453. doi: 10.1007/s11033-024-09414-8. Mol Biol Rep. 2024. PMID: 38536553
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
