miR-10b-5p rescues leaky gut linked with gastrointestinal dysmotility and diabetes
- PMID: 37723933
- PMCID: PMC10576606
- DOI: 10.1002/ueg2.12463
miR-10b-5p rescues leaky gut linked with gastrointestinal dysmotility and diabetes
Erratum in
-
Correction to miR-10b-5p rescues leaky gut linked with gastrointestinal dysmotility and diabetes.United European Gastroenterol J. 2024 Feb;12(1):163. doi: 10.1002/ueg2.12529. Epub 2024 Jan 5. United European Gastroenterol J. 2024. PMID: 38180731 Free PMC article. No abstract available.
Abstract
Background/aim: Diabetes has substantive co-occurrence with disorders of gut-brain interactions (DGBIs). The pathophysiological and molecular mechanisms linking diabetes and DGBIs are unclear. MicroRNAs (miRNAs) are key regulators of diabetes and gut dysmotility. We investigated whether impaired gut barrier function is regulated by a key miRNA, miR-10b-5p, linking diabetes and gut dysmotility.
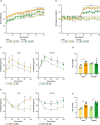
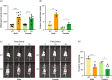
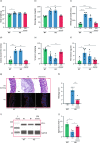
Methods: We created a new mouse line using the Mb3Cas12a/Mb3Cpf1 endonuclease to delete mir-10b globally. Loss of function studies in the mir-10b knockout (KO) mice were conducted to characterize diabetes, gut dysmotility, and gut barrier dysfunction phenotypes in these mice. Gain of function studies were conducted by injecting these mir-10b KO mice with a miR-10b-5p mimic. Further, we performed miRNA-sequencing analysis from colonic mucosa from mir-10b KO, wild type, and miR-10b-5p mimic injected mice to confirm (1) deficiency of miR-10b-5p in KO mice, and (2) restoration of miR-10b-5p after the mimic injection.
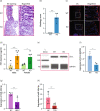
Results: Congenital loss of mir-10b in mice led to the development of hyperglycemia, gut dysmotility, and gut barrier dysfunction. Gut permeability was increased, but expression of the tight junction protein Zonula occludens-1 was reduced in the colon of mir-10b KO mice. Patients with diabetes or constipation- predominant irritable bowel syndrome, a known DGBI that is linked to leaky gut, had significantly reduced miR-10b-5p expression. Injection of a miR-10b-5p mimic in mir-10b KO mice rescued these molecular alterations and phenotypes.
Conclusions: Our study uncovered a potential pathophysiologic mechanism of gut barrier dysfunction that links both the diabetes and gut dysmotility phenotypes in mice lacking miR-10b-5p. Treatment with a miR-10b-5p mimic reversed the leaky gut, diabetic, and gut dysmotility phenotypes, highlighting the translational potential of the miR-10b-5p mimic.
Keywords: diabetic dysmotility; gastroparesis; intestinal barrier dysfunction; irritable bowel syndrome; microRNAs.
© 2023 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
This author discloses the following: Seungil Ro and the University of Nevada Reno Office of Technology Transfer (serial no. 62/837,988, filed 24 April 2019) have published a PCT International Patent WO/2020/219872 entitled “Methods and compositions of miR‐10 mimics and targets thereof.” Seungil Ro is an employee and a member of the board of directors of RosVivo Therapeutics. Rajan Singh and Se Eun Ha are members of the board of directors of RosVivo Therapeutics. The remaining authors disclose no conflicts.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

