Ebola Virus Uses Tunneling Nanotubes as an Alternate Route of Dissemination
- PMID: 37723997
- PMCID: PMC10651192
- DOI: 10.1093/infdis/jiad400
Ebola Virus Uses Tunneling Nanotubes as an Alternate Route of Dissemination
Abstract
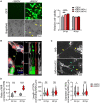
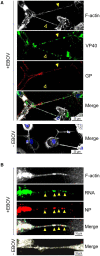
Ebola virus (EBOV) disease is marked by rapid virus replication and spread. EBOV enters the cell by macropinocytosis and replicates in the cytoplasm, and nascent virions egress from the cell surface to infect neighboring cells. Here, we show that EBOV uses an alternate route to disseminate: tunneling nanotubes (TNTs). TNTs, an actin-based long-range intercellular communication system, allows for direct exchange of cytosolic constituents between cells. Using live, scanning electron, and high-resolution quantitative 3-dimensional microscopy, we show that EBOV infection of primary human cells results in the enhanced formation of TNTs containing viral nucleocapsids. TNTs promote the intercellular transfer of nucleocapsids in the absence of live virus, and virus could replicate in cells devoid of entry factors after initial stall. Our studies suggest an alternate model of EBOV dissemination within the host, laying the groundwork for further investigations into the pathogenesis of filoviruses and, importantly, stimulating new areas of antiviral design.
Keywords: Ebola virus; spread; tunneling nanotubes.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Feldmann H, Sprecher A, Geisbert TW. Ebola. N Engl J Med 2020; 382:1832–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

