The gut-cardiovascular connection: new era for cardiovascular therapy
- PMID: 37724079
- PMCID: PMC10388818
- DOI: 10.1515/mr-2021-0002
The gut-cardiovascular connection: new era for cardiovascular therapy
Abstract
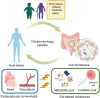
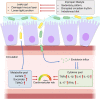
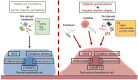
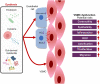
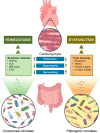
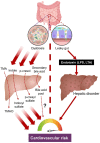
Our gut microbiome is constituted by trillions of microorganisms including bacteria, archaea and eukaryotic microbes. Nowadays, gut microbiome has been gradually recognized as a new organ system that systemically and biochemically interact with the host. Accumulating evidence suggests that the imbalanced gut microbiome contributes to the dysregulation of immune system and the disruption of cardiovascular homeostasis. Specific microbiome profiles and altered intestinal permeability are often observed in the pathophysiology of cardiovascular diseases. Gut-derived metabolites, toxins, peptides and immune cell-derived cytokines play pivotal roles in the induction of inflammation and the pathogenesis of dysfunction of heart and vasculature. Impaired crosstalk between gut microbiome and multiple organ systems, such as gut-vascular, heart-gut, gut-liver and brain-gut axes, are associated with higher cardiovascular risks. Medications and strategies that restore healthy gut microbiome might therefore represent novel therapeutic options to lower the incidence of cardiovascular and metabolic disorders.
Keywords: cardiovascular diseases; dysbiosis; endothelium; endotoxin; fecal microbiota transplantation; gut microbiome.
© 2021 Chak Kwong Cheng and Yu Huang, published by De Gruyter, Berlin/Boston.
Conflict of interest statement
Conflict of interest: The authors declare that there is no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
