The hemodynamic interplay between pulmonary ischemia-reperfusion injury and right ventricular function in lung transplantation: a translational porcine model
- PMID: 37724349
- PMCID: PMC11550898
- DOI: 10.1152/ajplung.00281.2022
The hemodynamic interplay between pulmonary ischemia-reperfusion injury and right ventricular function in lung transplantation: a translational porcine model
Abstract
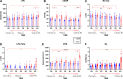
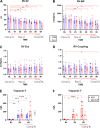
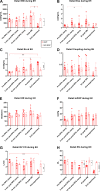
Lung transplantation (LTx) is a challenging procedure. Following the process of ischemia-reperfusion injury, the transplanted pulmonary graft might become severely damaged, resulting in primary graft dysfunction. In addition, during the intraoperative window, the right ventricle (RV) is at risk of acute failure. The interaction of right ventricular function with lung injury is, however, poorly understood. We aimed to address this interaction in a translational porcine model of pulmonary ischemia-reperfusion injury. Advanced pulmonary and hemodynamic assessment was used, including right ventricular pressure-volume loop analysis. The acute model was based on clamping and unclamping of the left lung hilus, respecting the different hemodynamic phases of a clinical lung transplantation. We found that forcing entire right ventricular cardiac output through a lung suffering from ischemia-reperfusion injury increased afterload (pulmonary vascular resistance from baseline to end experiment P < 0.0001) and induced right ventricular failure (RVF) in 5/9 animals. Notably, we identified different compensation patterns in failing versus nonfailing ventricles (arterial elastance P = 0.0008; stroke volume P < 0.0001). Furthermore, increased vascular pressure and flow produced by the right ventricle resulted in higher pulmonary injury, as measured by ex vivo CT density (correlation: pressure r = 0.8; flow r = 0.85). Finally, RV ischemia as measured by troponin-T was negatively correlated with pulmonary injury (r = -0.76); however, troponin-T values did not determine RVF in all animals. In conclusion, we demonstrate a delicate balance between development of pulmonary ischemia-reperfusion injury and right ventricular function during lung transplantation. Furthermore, we provide a physiological basis for potential benefit of extracorporeal life support technology.NEW & NOTEWORTHY In contrast to the abundant literature of mechanical pulmonary artery clamping to increase right ventricular afterload, we developed a model adding a biological factor of pulmonary ischemia-reperfusion injury. We did not only focus on the right ventricular behavior, but also on the interaction with the injured lung. We are the first to describe this interaction while addressing the hemodynamic intraoperative phases of clinical lung transplantation.
Keywords: lung transplantation; porcine model; pulmonary ischemia-reperfusion injury; right ventricular function.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Van Slambrouck J, Van Raemdonck D, Vos R, Vanluyten C, Vanstapel A, Prisciandaro E, Willems L, Orlitová M, Kaes J, Jin X, Jansen Y, Verleden GM, Neyrinck AP, Vanaudenaerde BM, Ceulemans LJ. A focused review on primary graft dysfunction after clinical lung transplantation: a multilevel syndrome. Cells 11: 745, 2022. doi:10.3390/cells11040745. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

