Structural and functional analysis of salivary intercalated duct cells reveals a secretory phenotype
- PMID: 37724716
- PMCID: PMC10591963
- DOI: 10.1113/JP285104
Structural and functional analysis of salivary intercalated duct cells reveals a secretory phenotype
Abstract
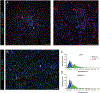
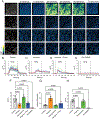
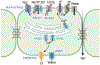
Currently, all salivary ducts (intercalated, striated and collecting) are assumed to function broadly in a similar manner, reclaiming ions that were secreted by the secretory acinar cells while preserving fluid volume and delivering saliva to the oral cavity. Nevertheless, there has been minimal investigation into the structural and functional differences between distinct types of salivary duct cells. Therefore, in this study, the expression profile of proteins involved in stimulus-secretion coupling, as well as the function of the intercalated duct (ID) and striated duct cells, was examined. Particular focus was placed on defining differences between distinct duct cell populations. To accomplish this, immunohistochemistry and in situ hybridization were utilized to examine the localization and expression of proteins involved in reabsorption and secretion of ions and fluid. Further, in vivo calcium imaging was employed to investigate cellular function. Based on the protein expression profile and functional data, marked differences between the IDs and striated ducts were observed. Specifically, the ID cells express proteins native to the secretory acinar cells while lacking proteins specifically expressed in the striated ducts. Further, the ID and striated duct cells display different calcium signalling characteristics, with the IDs responding to a neural stimulus in a manner similar to the acinar cells. Overall, our data suggest that the IDs have a distinct role in the secretory process, separate from the reabsorptive striated ducts. Instead, based on our evidence, the IDs express proteins found in secretory cells, generate calcium signals in a manner similar to acinar cells, and, therefore, are likely secretory cells. KEY POINTS: Current studies examining salivary intercalated duct cells are limited, with minimal documentation of the ion transport machinery and the overall role of the cells in fluid generation. Salivary intercalated duct cells are presumed to function in the same manner as other duct cells, reclaiming ions, maintaining fluid volume and delivering the final saliva to the oral cavity. Here we systematically examine the structure and function of the salivary intercalated duct cells using immunohistochemistry, in situ hybridization and by monitoring in vivo Ca2+ dynamics. Structural data revealed that the intercalated duct cells lack proteins vital for reabsorption and express proteins necessary for secretion. Ca2+ dynamics in the intercalated duct cells were consistent with those observed in secretory cells and resulted from GPCR-mediated IP3 production.
Keywords: fluid secretion; intracellular calcium; intravital imaging; salivary duct; salivary gland.
© 2023 The Authors. The Journal of Physiology © 2023 The Physiological Society.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

