Long-term follow-up results of multiparametric prostate MRI and the prognostic value of PI-RADS: a single-center retrospective cohort study
- PMID: 37724756
- PMCID: PMC11095067
- DOI: 10.4274/dir.2023.232414
Long-term follow-up results of multiparametric prostate MRI and the prognostic value of PI-RADS: a single-center retrospective cohort study
Abstract
Purpose: We aim to examine the long-term outcomes of patients who underwent multiparametric prostate magnetic resonance imaging (mp-MRI) for suspected prostate cancer (PCa), specifically based on their initial Prostate Imaging Reporting and Data System (PI-RADS) categories and various clinical factors. Our secondary aim is to evaluate the prognostic value of the PI-RADS through the National Comprehensive Cancer Network (NCCN) risk group distribution.
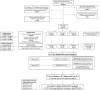
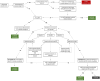
Methods: This research was conducted as a single-center retrospective cohort study in a tertiary care hospital. A total of 1,359 cases having at least one histopathological examination after the initial mp-MRI and/or adequate clinical/radiological follow-up data were included in the clinically significant PCa (cs-PCa) diagnosis-free survival analysis. Initial mp-MRI dates were accepted as the start of follow-up for the time-to-event analysis. The event was defined as cs-PCa diagnosis (International Society of Urological Pathology ≥2). Patients who were not diagnosed with cs-PCa during follow-up were censored according to predefined literature-based criteria at the end of the maximum follow-up duration with no reasonable suspicion of PCa and no biopsy indication. The impact of various factors on survival was assessed using a log-rank test and multivariable Cox regression. Subsequently, 394 cases diagnosed with PCa during follow-up were evaluated, based on initial PI-RADS categories and NCCN risk groups.
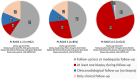
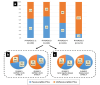
Results: Three main risk factors for cs-PCa diagnosis during follow-up were an initial PI-RADS 5 category, initial PI-RADS 4 category, and high MRI-defined PSA density (mPSAD), with average hazard ratios of 29.52, 14.46, and 3.12, respectively. The PI-RADS 3 category, advanced age group, and biopsy-naïve status were identified as additional risk factors (hazard ratios: 2.03, 1.54-1.98, and 1.79, respectively). In the PI-RADS 1-2 cohort, 1, 3, and 5-year cs-PCa diagnosis-free survival rates were 99.1%, 96.5%, and 93.8%, respectively. For the PI-RADS 3 cohort, 1, 3, and 5-year cs-PCa diagnosis-free survival rates were 94.9%, 90.9%, and 89.1%, respectively. For the PI-RADS 4 cohort, 1, 3, and 5-year cs-PCa diagnosis-free survival rates were 56.6%, 55.1%, and 55.1%, respectively. These rates were found to all be 24.2% in the PI-RADS 5 cohort. Considering the 394 cases diagnosed with PCa during follow-up, PI-RADS ≥4 cases were more likely to harbor unfavorable PCa compared to PI-RADS ≤3 cases (P < 0.001). In the PI-RADS 3 subgroup analysis, a low mPSAD (<0.15 ng/mL2) was found to be a protective prognostic factor against unfavorable PCa (P = 0.005).
Conclusion: The PI-RADS category has a significant impact on patient management and provides important diagnostic and prognostic information. Higher initial PI-RADS categories are associated with decreased follow-up losses, a shorter time to PCa diagnosis, increased biopsy rates, a higher likelihood of developing cs-PCa during follow-up, and a worse PCa prognosis. Combining mPSAD with PI-RADS categories could enhance diagnostic stratification in the identification of cs-PCa.
Keywords: Prostatic neoplasm; biopsy; diagnosis; follow-up studies; multiparametric magnetic resonance imaging; prognosis.
Conflict of interest statement
Hacettepe University Department of Radiology is one of the 20 partners of the Pro-Cancer-I project as a data provider. D.A. is the principal investigator, and A.D.K., M.K., and M.N.Ö. are researchers for the ProCAncer-I project, which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no: 952159. Other authors (Ö.Ö., M.A., Y.Y., V.G., M.S.Y., and B.A.) declare no conflict of interest.
Figures


Similar articles
-
Follow-up on patients with initial negative mpMRI target and systematic biopsy for PI-RADS ≥ 3 lesions - an EAU-YAU study enhancing prostate cancer detection.Prostate Cancer Prostatic Dis. 2025 Jun;28(2):435-443. doi: 10.1038/s41391-024-00904-1. Epub 2024 Nov 5. Prostate Cancer Prostatic Dis. 2025. PMID: 39501078
-
Diagnostic Accuracy and Prognostic Value of Serial Prostate Multiparametric Magnetic Resonance Imaging in Men on Active Surveillance for Prostate Cancer.Eur Urol Oncol. 2022 Oct;5(5):537-543. doi: 10.1016/j.euo.2020.11.007. Epub 2021 Jan 19. Eur Urol Oncol. 2022. PMID: 33483265
-
Enhancing Prostate Cancer Detection in PI-RADS 3 Cases: An In-depth Analysis of Radiological Indicators from Multiparametric MRI.Rofo. 2025 Jun;197(6):669-681. doi: 10.1055/a-2374-2531. Epub 2024 Sep 5. Rofo. 2025. PMID: 39236741 English.
-
Prostate Cancer Detection Percentages of Repeat Biopsy in Patients with Positive Multiparametric Magnetic Resonance Imaging (Prostate Imaging Reporting and Data System/Likert 3-5) and Negative Initial Biopsy. A Mini Systematic Review.Eur Urol. 2022 Nov;82(5):452-457. doi: 10.1016/j.eururo.2022.07.025. Epub 2022 Aug 18. Eur Urol. 2022. PMID: 35985901
-
Multiparametric MRI in detection and staging of prostate cancer.Dan Med J. 2017 Feb;64(2):B5327. Dan Med J. 2017. PMID: 28157066 Review.
Cited by
-
Long-Term Risk of Clinically Significant Prostate Cancer in Biopsy-Negative Patients With Baseline Biparametric Prostate MRI.J Magn Reson Imaging. 2025 Jun;61(6):2425-2432. doi: 10.1002/jmri.29668. Epub 2024 Nov 27. J Magn Reson Imaging. 2025. PMID: 39601084 Free PMC article.
References
-
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479–505. - PubMed
-
- Hernandez DJ, Nielsen ME, Han M, Partin AW. Contemporary evaluation of the D’amico risk classification of prostate cancer. Urology. 2007;70(5):931–935. - PubMed
-
- Gupta RT, Mehta KA, Turkbey B, Verma S. PI‐RADS: Past, present, and future. J Magn Reson Imaging. 2020;52(1):33–53. - PubMed
-
- Mottet N, van den Bergh RC, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

