Early antibody treatment, inflammation, and risk of post-COVID conditions
- PMID: 37724870
- PMCID: PMC10653913
- DOI: 10.1128/mbio.00618-23
Early antibody treatment, inflammation, and risk of post-COVID conditions
Erratum in
-
Erratum for Gebo et al., "Early antibody treatment, inflammation, and risk of post-COVID conditions".mBio. 2024 Jan 16;15(1):e0297923. doi: 10.1128/mbio.02979-23. Epub 2023 Dec 14. mBio. 2024. PMID: 38095433 Free PMC article. No abstract available.
Abstract
Approximately 20% of individuals infected with SARS-CoV-2 experienced long-term health effects, as defined PCC. However, it is unknown if there are any early biomarkers associated with PCC or whether early intervention treatments may decrease the risk of PCC. In a secondary analysis of a randomized clinical trial, this study demonstrates that among outpatients with SARS-CoV-2, increased IL-6 at time of infection is associated with increased odds of PCC. In addition, among individuals treated early, within 5 days of symptom onset, with COVID-19 convalescent plasma, there was a trend for decreased odds of PCC after adjusting for other demographic and clinical characteristics. Future treatment studies should be considered to evaluate the effect of early treatment and anti-IL-6 therapies on PCC development.
Keywords: COVID-19; COVID-19 serotherapy; chemokines; cytokines; interleukin-6; post-COVID condition (PCC); post-acute sequelae of COVID (PASC).
Conflict of interest statement
See Acknowledgments for conflicts of interest.
Figures
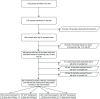

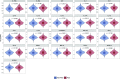
Comment in
-
Igniting the slow burn of post-COVID conditions.mBio. 2023 Oct 31;14(5):e0148923. doi: 10.1128/mbio.01489-23. Epub 2023 Sep 26. mBio. 2023. PMID: 37750708 Free PMC article.
References
-
- Centers for Disease Control and Prevention . COVID data tracker. Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed 11 March 2023
-
- Centers for Disease Control and Prevention . Long COVID or post-COVID conditions. Available from: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects. Accessed 11 March 2023
-
- Centers for Disease Control and Prevention . Post-COVID conditions: Overview for Healthcare providers. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-c.... Accessed 11 March 2023
-
- Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, Lekoubou A, Oh JS, Ericson JE, Ssentongo P, Chinchilli VM. 2021. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 4:e2128568. doi: 10.1001/jamanetworkopen.2021.28568 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
