Unravelling Surface Modification Strategies for Preventing Medical Device-Induced Thrombosis
- PMID: 37725037
- PMCID: PMC11468451
- DOI: 10.1002/adhm.202301039
Unravelling Surface Modification Strategies for Preventing Medical Device-Induced Thrombosis
Abstract
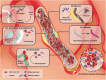
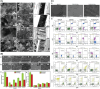
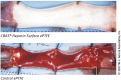
The use of biomaterials in implanted medical devices remains hampered by platelet adhesion and blood coagulation. Thrombus formation is a prevalent cause of failure of these blood-contacting devices. Although systemic anticoagulant can be used to support materials and devices with poor blood compatibility, its negative effects such as an increased chance of bleeding, make materials with superior hemocompatibility extremely attractive, especially for long-term applications. This review examines blood-surface interactions, the pathogenesis of clotting on blood-contacting medical devices, popular surface modification techniques, mechanisms of action of anticoagulant coatings, and discusses future directions in biomaterial research for preventing thrombosis. In addition, this paper comprehensively reviews several novel methods that either entirely prevent interaction between material surfaces and blood components or regulate the reaction of the coagulation cascade, thrombocytes, and leukocytes.
Keywords: medical devices; neoendothelialization; surface coatings; surface modification; thrombosis.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Hemocompatibility studies on a degradable polar hydrophobic ionic polyurethane (D-PHI).Acta Biomater. 2017 Jan 15;48:368-377. doi: 10.1016/j.actbio.2016.11.005. Epub 2016 Nov 3. Acta Biomater. 2017. PMID: 27818307
-
The blood compatibility challenge. Part 1: Blood-contacting medical devices: The scope of the problem.Acta Biomater. 2019 Aug;94:2-10. doi: 10.1016/j.actbio.2019.06.021. Epub 2019 Jun 18. Acta Biomater. 2019. PMID: 31226480 Review.
-
Heparin coatings for improving blood compatibility of medical devices.Adv Drug Deliv Rev. 2017 Mar;112:12-23. doi: 10.1016/j.addr.2016.12.002. Epub 2016 Dec 29. Adv Drug Deliv Rev. 2017. PMID: 28042080 Review.
-
Medical device-induced thrombosis: what causes it and how can we prevent it?J Thromb Haemost. 2015 Jun;13 Suppl 1:S72-81. doi: 10.1111/jth.12961. J Thromb Haemost. 2015. PMID: 26149053 Review.
-
Device thrombosis and pre-clinical blood flow models for assessing antithrombogenic efficacy of drug-device combinations.Adv Drug Deliv Rev. 2017 Mar;112:24-34. doi: 10.1016/j.addr.2016.07.009. Epub 2016 Aug 3. Adv Drug Deliv Rev. 2017. PMID: 27496706 Review.
Cited by
-
Role of chemistry in nature-inspired skin adhesives.Chem Sci. 2025 May 30;16(24):10665-10690. doi: 10.1039/d5sc01777g. eCollection 2025 Jun 18. Chem Sci. 2025. PMID: 40474951 Free PMC article. Review.
-
ISO 10993-4 Compliant Hemocompatibility Evaluation of Gellan Gum Hybrid Hydrogels for Biomedical Applications.Gels. 2024 Dec 13;10(12):824. doi: 10.3390/gels10120824. Gels. 2024. PMID: 39727582 Free PMC article.
-
Structure and Functional Characteristics of Novel Polyurethane/Ferrite Nanocomposites with Antioxidant Properties and Improved Biocompatibility for Vascular Graft Development.Polymers (Basel). 2025 Jan 9;17(2):152. doi: 10.3390/polym17020152. Polymers (Basel). 2025. PMID: 39861225 Free PMC article.
-
"Reactive" Chemical Strategy to Attain Substrate Independent "Liquid-Like" Omniphobic Solid Anti-Biofouling Coatings.Adv Funct Mater. 2024 Sep 4;34(36):2401387. doi: 10.1002/adfm.202401387. Epub 2024 Apr 18. Adv Funct Mater. 2024. PMID: 39678671 Free PMC article.
-
In vivo assessment of dual-function submicron textured nitric oxide releasing catheters in a 7-day rabbit model.Acta Biomater. 2024 May;180:372-382. doi: 10.1016/j.actbio.2024.04.009. Epub 2024 Apr 16. Acta Biomater. 2024. PMID: 38614415 Free PMC article.
References
-
- Bernard M., Jubeli E., Pungente M. D., Yagoubi N., Biomater. Sci. 2018, 6, 2025. - PubMed
-
- Kimmoun A., Oulehri W., Sonneville R., Grisot P. H., Zogheib E., Amour J., Aissaoui N., Megarbane B., Mongardon N., Renou A., Schmidt M., Besnier E., Delmas C., Dessertaine G., Guidon C., Nesseler N., Labro G., Rozec B., Pierrot M., Helms J., Bougon D., Chardonnal L., Medard A., Ouattara A., Girerd N., Lamiral Z., Borie M., Ajzenberg N., Levy B., Intensive Care Med. 2018, 44, 1460. - PubMed
-
- Murphy D. A., Hockings L. E., Andrews R. K., Aubron C., Gardiner E. E., Pellegrino V. A., Davis A. K., Transfus. Med. Rev. 2015, 29, 90. - PubMed
-
- Luyt C. E., Bréchot N., Demondion P., Jovanovic T., Hékimian G., Lebreton G., Nieszkowska A., Schmidt M., Trouillet J. L., Leprince P., Chastre J., Combes A., Intensive Care Med. 2016, 42, 897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

