Large extracellular vesicles derived from human regulatory macrophages (L-EVMreg) attenuate CD3/CD28-induced T-cell activation in vitro
- PMID: 37725101
- PMCID: PMC10663190
- DOI: 10.1007/s00109-023-02374-9
Large extracellular vesicles derived from human regulatory macrophages (L-EVMreg) attenuate CD3/CD28-induced T-cell activation in vitro
Abstract
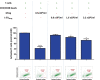
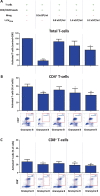
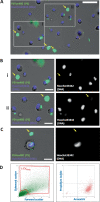
Macrophages belong to the innate immune system, and we have recently shown that in vitro differentiated human regulatory macrophages (Mreg) release large extracellular vesicles (L-EVMreg) with an average size of 7.5 μm which regulate wound healing and angiogenesis in vitro. The aim of this study was to investigate whether L-EVMreg also affect the CD3/CD28-mediated activation of T-cells. Mreg were differentiated using blood monocytes and L-EVMreg were isolated from culture supernatants by differential centrifugation. Activation of human T-cells was induced by CD3/CD28-coated beads in the absence or presence of Mreg or different concentrations of L-EVMreg. Inhibition of T-cell activation was quantified by flow cytometry and antibodies directed against the T-cell marker granzyme B. Phosphatidylserine (PS) exposure on the surface of Mreg and L-EVMreg was analyzed by fluorescence microscopy. Incubation of human lymphocytes with CD3/CD28 beads resulted in an increase of cell size, cell granularity, and number of granzyme B-positive cells (P < 0.05) which is indicative of T-cell activation. The presence of Mreg (0.5 × 106 Mreg/ml) led to a reduction of T-cell activation (number of granzyme B-positive cells; P < 0.001), and a similar but less pronounced effect was also observed when incubating activated T-cells with L-EVMreg (P < 0.05 for 3.2 × 106 L-EVMreg/ml). A differential analysis of the effects of Mreg and L-EVMreg on CD4+ and CD8+ T-cells showed an inhibition of CD4+ T-cells by Mreg (P < 0.01) and L-EVMreg (P < 0.05 for 1.6 × 106 L-EVMreg/ml; P < 0.01 for 3.2 × 106 L-EVMreg/ml). A moderate inhibition of CD8+ T-cells was observed by Mreg (P < 0.05) and by L-EVMreg (P < 0.01 for 1.6 × 106 L-EVMreg/ml and 3.2 × 106 L-EVMreg/ml). PS was restricted to confined regions of the Mreg surface, while L-EVMreg showed strong signals for PS in the exoplasmic leaflet. L-EVMreg attenuate CD3/CD28-mediated activation of CD4+ and CD8+ T-cells. L-EVMreg may have clinical relevance, particularly in the treatment of diseases associated with increased T-cell activity. KEY MESSAGES: Mreg release large extracellular vesicles (L-EVMreg) with an average size of 7.5 µm L-EVMreg exhibit phosphatidylserine positivity L-EVMreg suppress CD4+ and CD8+ T-cells L-EVMreg hold clinical potential in T-cell-related diseases.
Keywords: Large extracellular vesicles; Macrophages; Phosphatidylserine; T-cell activation.
© 2023. The Author(s).
Conflict of interest statement
The authors (MA, KZ, FF, RB) are involved in a pending patent (European Patent Office, Nr. 23153832.3 Mreg-derived vesicles). All other authors declare no competing interests.
Figures


References
-
- Weng Q, Wang Y, Xie Y, Yu X, Zhang S, Ge J, Li Z, Ye G, Guo J. Extracellular vesicles-associated tRNA-derived fragments (tRFs): biogenesis, biological functions, and their role as potential biomarkers in human diseases. J Mol Med (Berl) 2022;100:679–695. doi: 10.1007/s00109-022-02189-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

