Rehabilitation Interventions for Physical Capacity and Quality of Life in Adults With Post-COVID-19 Condition: A Systematic Review and Meta-Analysis
- PMID: 37725376
- PMCID: PMC10509723
- DOI: 10.1001/jamanetworkopen.2023.33838
Rehabilitation Interventions for Physical Capacity and Quality of Life in Adults With Post-COVID-19 Condition: A Systematic Review and Meta-Analysis
Abstract
Importance: Current rehabilitation guidelines for patients with post-COVID-19 condition (PCC) are primarily based on expert opinions and observational data, and there is an urgent need for evidence-based rehabilitation interventions to support patients with PCC.
Objective: To synthesize the findings of existing studies that report on physical capacity (including functional exercise capacity, muscle function, dyspnea, and respiratory function) and quality of life outcomes following rehabilitation interventions in patients with PCC.
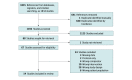
Data sources: A systematic electronic search was performed from January 2020 until February 2023, in MEDLINE, Scopus, CINAHL, and the Clinical Trials Registry. Key terms that were used to identify potentially relevant studies included long-covid, post-covid, sequelae, exercise therapy, rehabilitation, physical activity, physical therapy, and randomized controlled trial.
Study selection: This study included randomized clinical trials that compared respiratory training and exercise-based rehabilitation interventions with either placebo, usual care, waiting list, or control in patients with PCC.
Data extraction and synthesis: This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. A pairwise bayesian random-effects meta-analysis was performed using vague prior distributions. Risk of bias was assessed using the Cochrane risk of bias tool version 2, and the certainty of evidence was evaluated using the GRADE system by 2 independent researchers.
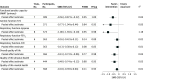
Main outcomes and measures: The primary outcome was functional exercise capacity, measured at the closest postintervention time point by the 6-minute walking test. Secondary outcomes were fatigue, lower limb muscle function, dyspnea, respiratory function, and quality of life. All outcomes were defined a priori. Continuous outcomes were reported as standardized mean differences (SMDs) with 95% credible intervals (CrIs) and binary outcomes were summarized as odds ratios with 95% CrIs. The between-trial heterogeneity was quantified using the between-study variance, τ2, and 95% CrIs.
Results: Of 1834 identified records, 1193 were screened, and 14 trials (1244 patients; 45% female participants; median [IQR] age, 50 [47 to 56] years) were included in the analyses. Rehabilitation interventions were associated with improvements in functional exercise capacity (SMD, -0.56; 95% CrI, -0.87 to -0.22) with moderate certainty in 7 trials (389 participants). These improvements had a 99% posterior probability of superiority when compared with current standard care. The value of τ2 (0.04; 95% CrI, 0.00 to 0.60) indicated low statistical heterogeneity. However, there was significant uncertainty and imprecision regarding the probability of experiencing exercise-induced adverse events (odds ratio, 1.68; 95% CrI, 0.32 to 9.94).
Conclusions and relevance: The findings of this systematic review and meta-analysis suggest that rehabilitation interventions are associated with improvements in functional exercise capacity, dyspnea, and quality of life, with a high probability of improvement compared with the current standard care; the certainty of evidence was moderate for functional exercise capacity and quality of life and low for other outcomes. Given the uncertainty surrounding the safety outcomes, additional trials with enhanced monitoring of adverse events are necessary.
Conflict of interest statement
Figures

References
-
- World Health Organization . Post COVID-19 condition (long COVID). December 7, 2022. Accessed February 25, 2023. https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-cond...
-
- Quinn KL, Katz GM, Bobos P, et al. . Understanding the post COVID-19 condition (long COVID) in adults and the expected burden for Ontario. Ontario COVID-19 Science Advisory Table . September 7, 2022. Accessed March 3, 2023. https://covid19-sciencetable.ca/sciencebrief/understanding-the-post-covi...
-
- Statistics Canada . Long-term symptoms in Canadian adults who tested positive for COVID-19 or suspected an infection, January 2020 to August 2022. October 17, 2022. Updated October 20, 2022. Accessed March 1, 2023. https://www150.statcan.gc.ca/n1/daily-quotidien/221017/dq221017b-eng.htm

