Advanced Age in Humans and Mouse Models of Glioblastoma Show Decreased Survival from Extratumoral Influence
- PMID: 37725593
- PMCID: PMC10690140
- DOI: 10.1158/1078-0432.CCR-23-0834
Advanced Age in Humans and Mouse Models of Glioblastoma Show Decreased Survival from Extratumoral Influence
Abstract
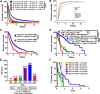
Purpose: Glioblastoma (GBM) is the most common aggressive primary malignant brain tumor in adults with a median age of onset of 68 to 70 years old. Although advanced age is often associated with poorer GBM patient survival, the predominant source(s) of maladaptive aging effects remains to be established. Here, we studied intratumoral and extratumoral relationships between adult patients with GBM and mice with brain tumors across the lifespan.
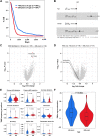
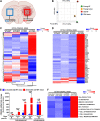
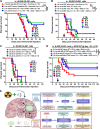
Experimental design: Electronic health records at Northwestern Medicine and the NCI SEER databases were evaluated for GBM patient age and overall survival. The commercial Tempus and Caris databases, as well as The Cancer Genome Atlas were profiled for gene expression, DNA methylation, and mutational changes with varying GBM patient age. In addition, gene expression analysis was performed on the extratumoral brain of younger and older adult mice with or without a brain tumor. The survival of young and old wild-type or transgenic (INK-ATTAC) mice with a brain tumor was evaluated after treatment with or without senolytics and/or immunotherapy.
Results: Human patients with GBM ≥65 years of age had a significantly decreased survival compared with their younger counterparts. While the intra-GBM molecular profiles were similar between younger and older patients with GBM, non-tumor brain tissue had a significantly different gene expression profile between young and old mice with a brain tumor and the eradication of senescent cells improved immunotherapy-dependent survival of old but not young mice.
Conclusions: This work suggests a potential benefit for combining senolytics with immunotherapy in older patients with GBM.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

