A randomized trial: The safety, pharmacokinetics and preliminary pharmacodynamics of ropivacaine oil delivery depot in healthy subjects
- PMID: 37725618
- PMCID: PMC10508611
- DOI: 10.1371/journal.pone.0291793
A randomized trial: The safety, pharmacokinetics and preliminary pharmacodynamics of ropivacaine oil delivery depot in healthy subjects
Abstract
Introduction: Ropivacaine oil delivery depot (RODD) can slowly release ropivacaine and block nerves for a long timejavascript:;. The aim of the present work was to investigate the safety, pharmacokinetics, and preliminary pharmacodynamics of RODD in subcutaneous injection among healthy subjects.
Methods: The abdomens of 3 subjects were subcutaneously administered with a single-needle RODD containing 12~30 mg of ropivacaine. The irritation, nerve blocking range and optimum dose were investigated. Forty-one subjects were divided into RODD groups containing 150, 230, 300, 350 and 400 mg of ropivacaine and a ropivacaine hydrochloride injection (RHI) 150 mg group. Multineedle subcutaneous injection of RODD or RHI was performed in the abdomens of the subjects. The primary endpoint was a safe dose or a maximum dose of ropivacaine (400 mg). Subjects' vital signs were observed; their blood was analyzed; their cardiovascular system and nervous systems were monitored, and their dermatological reactions were observed and scored. Second, the ropivacaine concentrations in plasma were determined, pharmacokinetic parameters were calculated, and the anesthetic effects of RODD were studied, including RODD onset time, duration and intensity of nerve block.
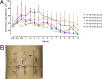
Results: Single-needle injection of RODD 24 mg was optimal for 3 subjects, and the range of nerve block was 42.5±20.8 mm. Multineedle subcutaneous injection of RODD in the abdomens of subjects was safe, and all adverse events were no more severe than grade II. The incidence rate of grade II adverse events, such as pain, and abnormal ST and ST-T segment changes on electrocardiography, was approximately 1%. The incidence rate of grade I adverse events, including erythema, papules, hypertriglyceridemia, and hypotension was greater than 10%. Erythema and papules were relieved after 24 h and disappeared after 72 h. Other adverse reactions disappeared after 7 days. The curve of ropivacaine concentration-time in plasma presented a bimodal profile. The results showed that ropivacaine was slowly released from the RODD. Compared with the 150 mg RHI group, Tmax was longer in the RODD groups. In particular, Tmax in the 400 mg RODD group was longer than that in the RHI group (11.8±4.6 h vs. 0.77±0.06 h). The Cmax in the 150 mg RODD group was lower than that in the 150 mg RHI group (0.35±0.09 vs. 0.58±0.13 μg·mL-1). In particular, the Cmax increased by 48% when the dose was increased by 2.6 times in the 400 mg group. Cmax, the AUC value and the intensity of the nerve block increased with increasing doses of RODD. Among them, the 400 mg RODD group presented the strongest nerve block (the percentage of level 2 and 3, 42.9%). The corresponding median onset time was 0.42 h, and the duration median was 35.7⁓47.7 h.
Conclusions: RODD has a sustained release effect. Compared with the RHI group, Tmax was delayed in the RODD groups, and the duration of nerve block was long. No abnormal reaction was found in the RODD group containing 400 mg of ropivacaine after subcutaneous injection among healthy subjects, suggesting that RODD was adequately safe.
Trial registration: Chictr.org: CTR2200058122; Chinadrugtrials.org: CTR20192280.
Copyright: © 2023 Lu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Pharmacodynamics, toxicology and toxicokinetics of ropivacaine oil delivery depot.BMC Anesthesiol. 2022 Apr 21;22(1):113. doi: 10.1186/s12871-022-01653-1. BMC Anesthesiol. 2022. PMID: 35448955 Free PMC article.
-
Safety, tolerability, pharmacokinetic, and pharmacodynamic of CZ1S injection for unilateral brachial plexus blockade in healthy Chinese adults: Double-blind, randomized, positive-controlled Phase I study.J Pharm Sci. 2025 Feb;114(2):1245-1253. doi: 10.1016/j.xphs.2025.01.008. Epub 2025 Jan 16. J Pharm Sci. 2025. PMID: 39826837 Clinical Trial.
-
A comparison of the pharmacodynamics and pharmacokinetics of bupivacaine, ropivacaine (with epinephrine) and their equal volume mixtures with lidocaine used for femoral and sciatic nerve blocks: a double-blind randomized study.Anesth Analg. 2009 Feb;108(2):641-9. doi: 10.1213/ane.0b013e31819237f8. Anesth Analg. 2009. PMID: 19151302 Clinical Trial.
-
Safety, tolerability, pharmacokinetics, and pharmacodynamics of recombinant human parathyroid hormone (1-34) in healthy Chinese subjects.Clin Ther. 2014 Jun 1;36(6):940-52. doi: 10.1016/j.clinthera.2014.03.015. Epub 2014 Apr 29. Clin Ther. 2014. PMID: 24793535 Clinical Trial.
-
Preliminary risk-benefit analysis of ropivacaine in labour and following surgery.Drug Saf. 1997 Jun;16(6):391-402. doi: 10.2165/00002018-199716060-00005. Drug Saf. 1997. PMID: 9241493 Review.
Cited by
-
Comparative Efficacy of Postoperative Pain Management Techniques Following Costal Cartilage Harvest: A Systematic Review and Network Meta-analysis.Aesthetic Plast Surg. 2025 Feb;49(3):929-949. doi: 10.1007/s00266-024-04430-2. Epub 2024 Nov 11. Aesthetic Plast Surg. 2025. PMID: 39527255 Free PMC article.
References
-
- Yutaka ODA. Ropivacaine: pharmacokinetics and toxicity. The journal of Japan Society for Clinical Anesthesia. 2009; 29(4): 519–27. 10.2199/jjsca.29.519 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

