Phenotypic Characterization of Congenital Hyperinsulinism Due to Novel Activating Glucokinase Mutations
- PMID: 37725835
- PMCID: PMC10658072
- DOI: 10.2337/db23-0465
Phenotypic Characterization of Congenital Hyperinsulinism Due to Novel Activating Glucokinase Mutations
Abstract
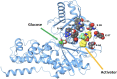
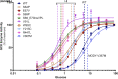
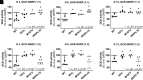
The importance of glucokinase (GK) in the regulation of insulin secretion has been highlighted by the phenotypes of individuals with activating and inactivating mutations in the glucokinase gene (GCK). Here we report 10 individuals with congenital hyperinsulinism (HI) caused by eight unique activating mutations of GCK. Six are novel and located near previously identified activating mutations sites. The first recognized episode of hypoglycemia in these patients occurred between birth and 24 years, and the severity of the phenotype was also variable. Mutant enzymes were expressed and purified for enzyme kinetics in vitro. Mutant enzymes had low glucose half-saturation concentration values and an increased enzyme activity index compared with wild-type GK. We performed functional evaluation of islets from the pancreata of three children with GCK-HI who required pancreatectomy. Basal insulin secretion in perifused GCK-HI islets was normal, and the response to glyburide was preserved. However, the threshold for glucose-stimulated insulin secretion in perifused glucokinase hyperinsulinism (GCK-HI) islets was decreased, and glucagon secretion was greatly suppressed. Our evaluation of novel GCK disease-associated mutations revealed that the detrimental effects of these mutations on glucose homeostasis can be attributed not only to a lowering of the glucose threshold of insulin secretion but also to a decreased counterregulatory glucagon secretory response.
Article highlights: Our evaluation of six novel and two previously published activating GCK mutations revealed that the detrimental effects of these mutations on glucose homeostasis can be attributed not only to a lowering of the glucose threshold of insulin secretion but also to a decreased counterregulatory glucagon secretory response. These studies provide insights into the pathophysiology of GCK-hyperinsulinism and the dual role of glucokinase in β-cells and α-cells to regulate glucose homeostasis.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures



Similar articles
-
Heterogeneity in phenotype of hyperinsulinism caused by activating glucokinase mutations: a novel mutation and its functional characterization.Clin Endocrinol (Oxf). 2017 Jun;86(6):778-783. doi: 10.1111/cen.13318. Epub 2017 Mar 27. Clin Endocrinol (Oxf). 2017. PMID: 28247534
-
A report of 2 new cases of MODY2 and review of the literature: implications in the search for type 2 diabetes drugs.Metabolism. 2013 Nov;62(11):1535-42. doi: 10.1016/j.metabol.2013.06.007. Epub 2013 Jul 24. Metabolism. 2013. PMID: 23890519 Review.
-
Nutrient sensing in pancreatic islets: lessons from congenital hyperinsulinism and monogenic diabetes.Ann N Y Acad Sci. 2018 Jan;1411(1):65-82. doi: 10.1111/nyas.13448. Epub 2017 Oct 16. Ann N Y Acad Sci. 2018. PMID: 29044608 Free PMC article. Review.
-
Severe persistent hyperinsulinemic hypoglycemia due to a de novo glucokinase mutation.Diabetes. 2004 Aug;53(8):2164-8. doi: 10.2337/diabetes.53.8.2164. Diabetes. 2004. PMID: 15277402
-
Activating glucokinase (GCK) mutations as a cause of medically responsive congenital hyperinsulinism: prevalence in children and characterisation of a novel GCK mutation.Eur J Endocrinol. 2008 Jul;159(1):27-34. doi: 10.1530/EJE-08-0203. Epub 2008 May 1. Eur J Endocrinol. 2008. PMID: 18450771
Cited by
-
Low-Level Mosaic GCK Mutations in Children With Diazoxide-Unresponsive Congenital Hyperinsulinism.J Clin Endocrinol Metab. 2025 Jun 17;110(7):1923-1928. doi: 10.1210/clinem/dgae713. J Clin Endocrinol Metab. 2025. PMID: 39382384 Free PMC article.
-
Genetic Variations in Hyperinsulinemic Hypoglycemia: Active versus Inactive Mutations.Diabetes Metab Syndr Obes. 2024 Nov 26;17:4439-4452. doi: 10.2147/DMSO.S482056. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39619217 Free PMC article. Review.
-
Mild Congenital Hyperinsulinism Caused by Mutation in Human Glucokinase Gene.JCEM Case Rep. 2024 Dec 4;2(12):luae226. doi: 10.1210/jcemcr/luae226. eCollection 2024 Dec. JCEM Case Rep. 2024. PMID: 39659388 Free PMC article.
-
Functional and Mechanistic Explanation for the Unique Clinical Success of the Glucokinase Activator Dorzagliatin in the Treatment of Type 2 Diabetes.Diabetes. 2025 Aug 1;74(8):1374-1384. doi: 10.2337/db25-0066. Diabetes. 2025. PMID: 40272935 Free PMC article.
-
Emerging approaches for the development of artificial islets.Smart Med. 2024 Mar 7;3(2):e20230042. doi: 10.1002/SMMD.20230042. eCollection 2024 Jun. Smart Med. 2024. PMID: 39188698 Free PMC article. Review.
References
-
- Davis EA, Cuesta-Muñoz A, Raoul M, et al. Mutants of glucokinase cause hypoglycaemia- and hyperglycaemia syndromes and their analysis illuminates fundamental quantitative concepts of glucose homeostasis. Diabetologia 1999;42:1175–1186 - PubMed
-
- Njølstad PR, Søvik O, Cuesta-Muñoz A, et al. Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med 2001;344:1588–1592 - PubMed
-
- Vionnet N, Stoffel M, Takeda J, et al. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature 1992;356:721–722 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

