Sleep consolidates stimulus-response learning
- PMID: 37726140
- PMCID: PMC10547380
- DOI: 10.1101/lm.053753.123
Sleep consolidates stimulus-response learning
Abstract
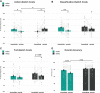
Performing a motor response to a sensory stimulus creates a memory trace whose behavioral correlates are classically investigated in terms of repetition priming effects. Such stimulus-response learning entails two types of associations that are partly independent: (1) an association between the stimulus and the motor response and (2) an association between the stimulus and the classification task in which it is encountered. Here, we tested whether sleep supports long-lasting stimulus-response learning on a task requiring participants (1) for establishing stimulus-classification associations to classify presented objects along two different dimensions ("size" and "mechanical") and (2) as motor response (action) to respond with either the left or right index finger. Moreover, we examined whether strengthening of stimulus-classification associations is preferentially linked to nonrapid eye movement (non-REM) sleep and strengthening of stimulus-action associations to REM sleep. We tested 48 healthy volunteers in a between-subjects design comparing postlearning retention periods of nighttime sleep versus daytime wakefulness. At postretention testing, we found that sleep supports consolidation of both stimulus-action and stimulus-classification associations, as indicated by increased reaction times in "switch conditions"; that is, when, at test, the acutely instructed classification task and/or correct motor response for a given stimulus differed from that during original learning. Polysomnographic recordings revealed that both kinds of associations were correlated with non-REM spindle activity. Our results do not support the view of differential roles for non-REM and REM sleep in the consolidation of stimulus-classification and stimulus-action associations, respectively.
© 2023 Miao et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Achermann P, Werth E, Dijk D-J, Borbely A. 1996. Time course of sleep inertia after nighttime and daytime sleep episodes. Arch Ital Biol 134: 109–119. - PubMed
-
- Ayoub A, Aumann D, Hörschelmann A, Kouchekmanesch A, Paul P, Born J, Marshall L. 2013. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep 36: 905–911. 10.5665/sleep.2722 - DOI - PMC - PubMed
-
- Baayen RH, Davidson DJ, Bates DM. 2008. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang 59: 390–412. 10.1016/j.jml.2007.12.005 - DOI
-
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. 10.18637/jss.v067.i01 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
