Hydrophobin Bilayer as Water Impermeable Protein Membrane
- PMID: 37726241
- PMCID: PMC10552762
- DOI: 10.1021/acs.langmuir.3c01006
Hydrophobin Bilayer as Water Impermeable Protein Membrane
Abstract
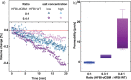
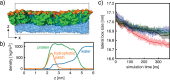
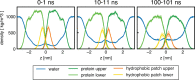
One of the most important properties of membranes is their permeability to water and other small molecules. A targeted change in permeability allows the passage of molecules to be controlled. Vesicles made of membranes with low water permeability are preferable for drug delivery, for example, because they are more stable and maintain the drug concentration inside. This study reports on the very low water permeability of pure protein membranes composed of a bilayer of the amphiphilic protein hydrophobin HFBI. Using a droplet interface bilayer setup, we demonstrate that HFBI bilayers are essentially impermeable to water. HFBI bilayers withstand far larger osmotic pressures than lipid membranes. Only by disturbing the packing of the proteins in the HFBI bilayer is a measurable water permeability induced. To investigate possible molecular mechanisms causing the near-zero permeability, we used all-atom molecular dynamics simulations of various HFBI bilayer models. The simulations suggest that the experimental HFBI bilayer permeability is compatible neither with a lateral honeycomb structure, as found for HFBI monolayers, nor with a residual oil layer within the bilayer or with a disordered lateral packing similar to the packing in lipid bilayers. These results suggest that the low permeabilities of HFBI and lipid bilayers rely on different mechanisms. With their extremely low but adaptable permeability and high stability, HFBI membranes could be used as an osmotic pressure-insensitive barrier in situations where lipid membranes fail such as desalination membranes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Kali R.; Andini E.; Milner S. T. Molecular Dynamics Simulation Based Design of Biomimetic Membrane with Artificial Water Channels. J. Membr. Sci. 2021, 630, 119279. 10.1016/j.memsci.2021.119279. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

