CSE reduces OTUD4 triggering lung epithelial cell apoptosis via PAI-1 degradation
- PMID: 37726265
- PMCID: PMC10509146
- DOI: 10.1038/s41419-023-06131-1
CSE reduces OTUD4 triggering lung epithelial cell apoptosis via PAI-1 degradation
Abstract
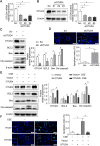
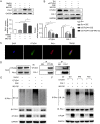
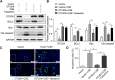
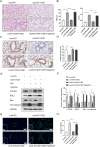
Ovarian tumor family deubiquitinase 4 (OTUD4), a member of the OTU deubiquitinating enzyme, is implicated to decrease in cancer to regulate cell apoptosis. However, the role of OTUD4 in cigarette smoke induced epithelial cell apoptosis and its mechanism have not been elucidated. In this study, we showed that OTUD4 protein reduced in CSE treated mice and airway epithelial cells. OTUD4 silence aggravated cell apoptosis and emphysematous change in the lung tissue of cigarette smoke extract (CSE) treated mice. Additionally, restoration of OTUD4 in the lung of mice alleviated CSE induced apoptosis and emphysematous morphology change. The effect of OTUD4 on cell apoptosis was also confirmed in vitro. Through protein profile screening, we identified that OTUD4 may interact with plasminogen activator inhibitor 1(PAI-1). We further confirmed that OTUD4 interacted with PAI-1 for de-ubiquitination and inhibiting CSE induced PAI-1 degradation. Furthermore, the protective role of OTUD4 in airway epithelial cells apoptosis was blocked by PAI-1 deactivation. Taken together, our data suggest that OTUD4 regulates cigarette smoke (CS)-triggered airway epithelial cell apoptosis via modulating PAI-1 degradation. Targeting OUTD4/PAI-1 signaling might potentially provide a therapeutic target against the lung cell apoptosis in cigarette smoke (CS)-induced emphysema.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Heron M. Deaths: leading causes for 2008. Natl Vital– Stat Rep. : Cent Dis Control Prev, Natl Cent Health Stat, Natl Vital– Stat Syst. 2012;60:1–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

