Maternal and paternal obesity differentially reprogram the ovarian mitochondrial biogenesis of F1 female rats
- PMID: 37726284
- PMCID: PMC10509203
- DOI: 10.1038/s41598-023-42468-5
Maternal and paternal obesity differentially reprogram the ovarian mitochondrial biogenesis of F1 female rats
Abstract
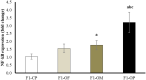
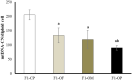
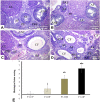
Obesity has harmful consequences on reproductive outcomes and the rapid increase in obesity is assumed to be influenced by epigenetics and trans-generation effects. Our study aimed to explore the effect of maternal and/or paternal obesity on the ovarian tissues of the first-generation female offspring in rats. The study was conducted on 40 adult Wistar albino rats (20 males and 20 females). Obesity was induced by feeding them an obesogenic diet for 3 months. The pregnancy was induced in the females by mating with males in four combinations: healthy mother with healthy father (control parents, CP), healthy mother with obese fathers (OF), obese mothers with healthy father (OM), and obese mother with obese father (obese parents, OP). After delivery, the female offspring at two months were sacrificed, and the blood and ovarian tissues were collected to assess the studied parameters. Our result showed differential impacts of maternal and paternal obesity on the ovarian health of the female offspring. The female offspring of obese OM or OP showed early signs of obesity. These metabolic abnormalities were associated with signs of ovarian lesions, impaired folliculogenesis, and decreased oocyte quality and also showed significant alterations in mitochondrial biogenesis, redox status, inflammation, and microRNAs expression (miR-149 and miR-494). In conclusion, altered ovarian expression of microRNAs and associated impaired mitochondrial biogenesis pathways may be the root causes for the observed intergeneration transmission of the obesogenic phenotype.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Abd El-aty NS, et al. Overweight and obesity prevalence among upper Egypt primary schools' children using Egyptian and CDC growth charts. Appl. Nurs. Res. 2020;56:151346. - PubMed
-
- Aktar N, Qureshi NK, Ferdous HS. Obesity: A review of pathogenesis and management strategies in adult. Delta Med. Coll. J. 2017;5(1):35–48.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

