Prosaposin maintains lipid homeostasis in dopamine neurons and counteracts experimental parkinsonism in rodents
- PMID: 37726325
- PMCID: PMC10509278
- DOI: 10.1038/s41467-023-41539-5
Prosaposin maintains lipid homeostasis in dopamine neurons and counteracts experimental parkinsonism in rodents
Abstract
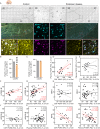
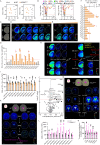
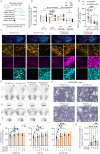
Prosaposin (PSAP) modulates glycosphingolipid metabolism and variants have been linked to Parkinson's disease (PD). Here, we find altered PSAP levels in the plasma, CSF and post-mortem brain of PD patients. Altered plasma and CSF PSAP levels correlate with PD-related motor impairments. Dopaminergic PSAP-deficient (cPSAPDAT) mice display hypolocomotion and depression/anxiety-like symptoms with mildly impaired dopaminergic neurotransmission, while serotonergic PSAP-deficient (cPSAPSERT) mice behave normally. Spatial lipidomics revealed an accumulation of highly unsaturated and shortened lipids and reduction of sphingolipids throughout the brains of cPSAPDAT mice. The overexpression of α-synuclein via AAV lead to more severe dopaminergic degeneration and higher p-Ser129 α-synuclein levels in cPSAPDAT mice compared to WT mice. Overexpression of PSAP via AAV and encapsulated cell biodelivery protected against 6-OHDA and α-synuclein toxicity in wild-type rodents. Thus, these findings suggest PSAP may maintain dopaminergic lipid homeostasis, which is dysregulated in PD, and counteract experimental parkinsonism.
© 2023. Springer Nature Limited.
Conflict of interest statement
J.L., L.U.W., and H.B. are employees of Sinfonia Biotherapeutics AB, focusing on encapsulated-cell biodelivery therapy. All other authors declare no competing interests.
Figures



References
-
- Poewe W, et al. Parkinson disease. Nat. Rev. Dis. Prim. 2017;3:17013. - PubMed
-
- Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548–560. - PubMed
-
- Vijiaratnam N, Simuni T, Bandmann O, Morris HR, Foltynie T. Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol. 2021;20:559–572. - PubMed
-
- Breiden B, Sandhoff K. Lysosomal glycosphingolipid storage diseases. Annu. Rev. Biochem. 2019;88:461–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

