A Comprehensive Literature Review on Managing Systemic Lupus Erythematosus: Addressing Cardiovascular Disease Risk in Females and Its Autoimmune Disease Associations
- PMID: 37727166
- PMCID: PMC10505685
- DOI: 10.7759/cureus.43725
A Comprehensive Literature Review on Managing Systemic Lupus Erythematosus: Addressing Cardiovascular Disease Risk in Females and Its Autoimmune Disease Associations
Abstract
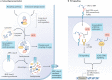
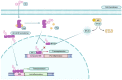
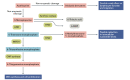
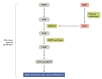
This review aimed to evaluate the mechanism of premature cardiovascular disease (CVD) in systemic lupus erythematosus (SLE) patients, particularly in the female population, and emphasize the need for early management interventions; explore the association between SLE and two autoimmune diseases, myasthenia gravis (MG) and antiphospholipid antibody syndrome (APS), and their management strategies; and evaluate the effectiveness of pharmacological and non-pharmacological interventions in managing SLE, focusing on premenopausal females, females of childbearing age, and pregnant patients. We conducted a comprehensive literature review to achieve these objectives using various databases, including PubMed, Google Scholar, and Cochrane. The collected data were analyzed and synthesized to provide an evidence-based overview of SLE, its management strategies as an independent disease, and some disease associations. The treatment should be focused on remission, preventing organ damage, and improving the overall quality of life (QOL). Extensive emphasis should also be focused on diagnosing SLE and concurrent underlying secondary diseases timely and managing them appropriately.
Keywords: adverse pregnancy outcomes; antiphospholipid antibody syndrome; cardiac manifestations; myasthenia gravis; serum biomarkers; systemic lupus erythematosus; therapeutic interventions.
Copyright © 2023, Dar et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Jakes RW, Bae SC, Louthrenoo W, Mok CC, Navarra SV, Kwon N. Arthritis Care Res (Hoboken) 2012;64:159–168. - PubMed
-
- Manifestations of systemic lupus erythematosus. Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3391953/ Maedica (Bucur) 2011;6:330–336. - PMC - PubMed
-
- Review article: systemic lupus erythematosus: a review for anesthesiologists. Ben-Menachem E. Anesth Analg. 2010;111:665–676. - PubMed
-
- Systemic lupus erythematosus and accelerated atherosclerosis. Hahn BH. N Engl J Med. 2003;349:2379–2380. - PubMed
-
- Monitoring systemic lupus erythematosus in standard clinical care. Petri M. Best Pract Res Clin Rheumatol. 2007;21:687–697. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
