Twenty-Month Regression Following Concurrent Conventional Whole-Brain Irradiation and Chemoimmunotherapy for ≥3.8 cm Cerebellar Metastasis From Small Cell Lung Cancer
- PMID: 37727186
- PMCID: PMC10506730
- DOI: 10.7759/cureus.43759
Twenty-Month Regression Following Concurrent Conventional Whole-Brain Irradiation and Chemoimmunotherapy for ≥3.8 cm Cerebellar Metastasis From Small Cell Lung Cancer
Abstract
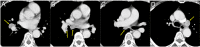
Standard whole-brain radiotherapy (WBRT) alone for large brain metastases (BMs) from small cell lung cancer (SCLC) has limited efficacy and durability, and stereotactic radiosurgery (SRS) alone for symptomatic posterior fossa BMs >3 cm with satellite lesions is challenging. Herein, we describe the case of a 73-year-old female presenting with treatment-naïve SCLC and 15 symptomatic multiple BMs, including a ≥3.8-cm cerebellar mass (≥17.7 cm3) and two adjacent lesions; otherwise, the SCLC was confined to the thorax. The patient was initially treated concurrently with conventional WBRT (30 Gy in 10 fractions) without boost and chemoimmunotherapy (CIT) consisting of carboplatin, etoposide, and atezolizumab. Atezolizumab was excluded during irradiation. Five months after WBRT, the large cerebellar lesion had remarkably regressed, and the smaller lesions (≤17 mm) showed complete responses (CRs) without local progression at 20 months. However, six and 16 months after WBRT, the thoracic lesions had progressed, and although amrubicin was administered, four new BMs, including pons involvement, had developed, respectively. Despite the CRs of the four BMs following SRS (49.6 Gy in eight fractions) and the sustained regression of the thoracic lesions, meningeal dissemination and multiple new BMs were evident 3.5 months post-SRS. The small remnant of the large BM and/or newly developed BMs abutting the cerebrospinal fluid (CSF) space could have led to CSF dissemination, the presumed cause of the patient's death. Taken together, concurrent chemo-WBRT and subsequent CIT can provide excellent and durable tumor responses for SCLC BMs, but may not be fully sufficient for BMs ≥3.8 cm. Therefore, in cases with large lesions, focal dose escalation of the large lesions, consolidative thoracic radiotherapy, and dose de-escalation in the macroscopically unaffected brain region may prevent or attenuate CSF dissemination, new BM development, and adverse effects and thus should be considered.
Keywords: brain metastases; chemoimmunotherapy; consolidative thoracic radiotherapy; extensive stage; large tumor; multi-fraction; pd-l1 inhibitor; small cell lung cancer; stereotactic radiosurgery; whole-brain radiotherapy.
Copyright © 2023, Ohtakara et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Update 2021: management of small cell lung cancer. Tariq S, Kim SY, Monteiro de Oliveira Novaes J, Cheng H. Lung. 2021;199:579–587. - PubMed
-
- Outcome and prognostic factors in patients with brain metastases from small-cell lung cancer treated with whole brain radiotherapy. Bernhardt D, Adeberg S, Bozorgmehr F, et al. J Neurooncol. 2017;134:205–212. - PubMed
-
- Stereotactic radiosurgery for brain metastases from newly diagnosed small cell lung cancer: practice patterns and outcomes. Jiang W, Haque W, Verma V, Butler B, Teh BS. Acta Oncol. 2019;58:491–498. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
