Host specific adaptations of Ligilactobacillus aviarius to poultry
- PMID: 37727231
- PMCID: PMC10505982
- DOI: 10.1016/j.crmicr.2023.100199
Host specific adaptations of Ligilactobacillus aviarius to poultry
Abstract
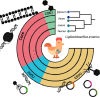
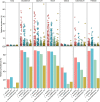
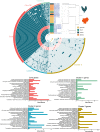
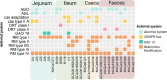
The genus Ligilactobacillus encompasses species adapted to vertebrate hosts and fermented food. Their genomes encode adaptations to the host lifestyle. Reports of gut microbiota from chicken and turkey gastrointestinal tract have shown a high persistence of Ligilactobacillus aviarius along the digestive system compared to other species found in the same host. However, its adaptations to poultry as a host has not yet been described. In this work, the pan-genome of Ligilactobacillus aviarius was explored to describe the functional adaptability to the gastrointestinal environment. The core genome is composed of 1179 gene clusters that are present at least in one copy that codifies to structural, ribosomal and biogenesis proteins. The rest of the identified regions were classified into three different functional clusters of orthologous groups (clusters) that codify carbohydrate metabolism, envelope biogenesis, viral defence mechanisms, and mobilome inclusions. The pan-genome of Ligilactobacillus aviarius is a closed pan-genome, frequently found in poultry and highly prevalent across chicken faecal samples. The genome of L. aviarius codifies different clusters of glycoside hydrolases and glycosyltransferases that mediate interactions with the host cells. Accessory features, such as antiviral mechanisms and prophage inclusions, variate amongst strains from different GIT sections. This information provides hints about the interaction of this species with viral particles and other bacterial species. This work highlights functional adaptability traits present in L. aviarius that make it a dominant key member of the poultry gut microbiota and enlightens the convergent ecological relation of this species to the poultry gut environment.
Keywords: Chicken; Ligilactobacillus aviarius; Pan-genome; Poultry.
© 2023 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Amato K.R., J G.S., Song S.J., Nute M., Metcalf J.L., Thompson L.R., Morton J.T., Amir A., V J.M., Humphrey G., Gogul G., Gaffney J., A L.B., G A.O.B., F P.C., Di Fiore A., N J.D., T L.G., Gomez A., Kowalewski M.M., R J.L., Link A., M L.S., Tecot S., B A.W., K E.N., R M.S., Knight R., S R.L. Evolutionary trends in host physiology outweigh dietary niche in structuring primate gut microbiomes. ISME J. 2019;13(3):576–587. doi: 10.1038/s41396-018-0175-0. - DOI - PMC - PubMed
-
- Asnicar F., Thomas A.M., Beghini F., Mengoni C., Manara S., Manghi P., Zhu Q., Bolzan M., Cumbo F., May U., Sanders J.G., Zolfo M., Kopylova E., Pasolli E., Knight R., Mirarab S., Huttenhower C., Segata N. Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat. Commun. 2020;11(1):2500. doi: 10.1038/s41467-020-16366-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

