Modelling the pathology and treatment of cardiac fibrosis in vascularised atrial and ventricular cardiac microtissues
- PMID: 37727305
- PMCID: PMC10506403
- DOI: 10.3389/fcvm.2023.1156759
Modelling the pathology and treatment of cardiac fibrosis in vascularised atrial and ventricular cardiac microtissues
Abstract
Introduction: Recent advances in human cardiac 3D approaches have yielded progressively more complex and physiologically relevant culture systems. However, their application in the study of complex pathological processes, such as inflammation and fibrosis, and their utility as models for drug development have been thus far limited.
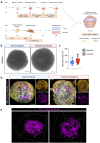
Methods: In this work, we report the development of chamber-specific, vascularised human induced pluripotent stem cell-derived cardiac microtissues, which allow for the multi-parametric assessment of cardiac fibrosis.
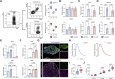
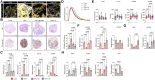
Results: We demonstrate the generation of a robust vascular system in the microtissues composed of endothelial cells, fibroblasts and atrial or ventricular cardiomyocytes that exhibit gene expression signatures, architectural, and electrophysiological resemblance to in vivo-derived anatomical cardiac tissues. Following pro-fibrotic stimulation using TGFβ, cardiac microtissues recapitulated hallmarks of cardiac fibrosis, including myofibroblast activation and collagen deposition. A study of Ca2+ dynamics in fibrotic microtissues using optical mapping revealed prolonged Ca2+ decay, reflecting cardiomyocyte dysfunction, which is linked to the severity of fibrosis. This phenotype could be reversed by TGFβ receptor inhibition or by using the BET bromodomain inhibitor, JQ1.
Discussion: In conclusion, we present a novel methodology for the generation of chamber-specific cardiac microtissues that is highly scalable and allows for the multi-parametric assessment of cardiac remodelling and pharmacological screening.
Keywords: 3D cardiac microtissues; cardiac fibrosis; cardiomyocytes; induced pluripotent stem cells; tissue engineering.
© 2023 Reyat, di Maio, Grygielska, Pike, Kemble, Rodriguez-Romero, Simoglou Karali, Croft, Psaila, Simões, Rayes and Khan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

