Cardiomyopathy as cause of death in Duchenne muscular dystrophy: a longitudinal observational study
- PMID: 37727676
- PMCID: PMC10505954
- DOI: 10.1183/23120541.00176-2023
Cardiomyopathy as cause of death in Duchenne muscular dystrophy: a longitudinal observational study
Abstract
Background: Cardiomyopathy has become an important life-limiting factor since survival in Duchenne muscular dystrophy (DMD) has greatly increased with long-term ventilation and cough assistance. The aim of this study was to investigate the association between impaired left ventricular ejection fraction (LVEF) and survival.
Methods: In a >20-year observational study in patients with DMD (age ≥16 years) with at least three echocardiograms, the association between LVEF and survival and time to cardiac or non-cardiac death was investigated using Kaplan-Meier survival analysis and Cox regression (for LVEF).
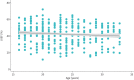
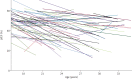
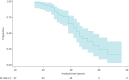
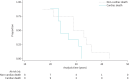
Results: In 67 DMD patients (430 echocardiograms), the decrease in LVEF over a mean±sd follow-up period of 9.1±5.1 years was -10.0±13.9% absolute, but LVEF progression varied widely. 84% were receiving an angiotensin-converting enzyme inhibitor and 54% a β-blocker at last follow-up with an LVEF of 37.5±12.4% at that time-point. Median (interquartile range) survival was 33 (25-40) years. 28 out of 67 (42%) of the cohort had died and LVEF was a significant negative predictor of survival (hazard ratio 0.95 (95% CI 0.91-0.99); p<0.007). Those who died of cardiac death (53% of known causes of death) had significantly lower LVEF at the time of death (LVEF -11.0% (95% CI -21.1- -0.9%); p=0.035) compared with non-cardiac death and tended to die at a younger age.
Conclusions: Cardiomyopathy with systolic heart failure is the leading cause of death and lower LVEF is an independent predictor of mortality at younger ages in patients with DMD. Patients with DMD appear to be undertreated with respect to heart failure drug therapy.
Copyright ©The authors 2023.
Conflict of interest statement
Conflicts of interest: The authors have no conflicts of interest to declare.
Figures


References
LinkOut - more resources
Full Text Sources
