Persistence of immunological memory as a potential correlate of long-term, vaccine-induced protection against Ebola virus disease in humans
- PMID: 37727795
- PMCID: PMC10505757
- DOI: 10.3389/fimmu.2023.1215302
Persistence of immunological memory as a potential correlate of long-term, vaccine-induced protection against Ebola virus disease in humans
Abstract
Introduction: In the absence of clinical efficacy data, vaccine protective effect can be extrapolated from animals to humans, using an immunological biomarker in humans that correlates with protection in animals, in a statistical approach called immunobridging. Such an immunobridging approach was previously used to infer the likely protective effect of the heterologous two-dose Ad26.ZEBOV, MVA-BN-Filo Ebola vaccine regimen. However, this immunobridging model does not provide information on how the persistence of the vaccine-induced immune response relates to durability of protection in humans.
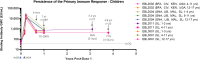
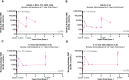
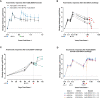
Methods and results: In both humans and non-human primates, vaccine-induced circulating antibody levels appear to be very stable after an initial phase of contraction and are maintained for at least 3.8 years in humans (and at least 1.3 years in non-human primates). Immunological memory was also maintained over this period, as shown by the kinetics and magnitude of the anamnestic response following re-exposure to the Ebola virus glycoprotein antigen via booster vaccination with Ad26.ZEBOV in humans. In non-human primates, immunological memory was also formed as shown by an anamnestic response after high-dose, intramuscular injection with Ebola virus, but was not sufficient for protection against Ebola virus disease at later timepoints due to a decline in circulating antibodies and the fast kinetics of disease in the non-human primates model. Booster vaccination within three days of subsequent Ebola virus challenge in non-human primates resulted in protection from Ebola virus disease, i.e. before the anamnestic response was fully developed.
Discussion: Humans infected with Ebola virus may benefit from the anamnestic response to prevent disease progression, as the incubation time is longer and progression of Ebola virus disease is slower as compared to non-human primates. Therefore, the persistence of vaccine-induced immune memory could be considered as a potential correlate of long-term protection against Ebola virus disease in humans, without the need for a booster.
Keywords: Ebola; correlate; immunological memory; persistence; protection; vaccine.
Copyright © 2023 McLean, Dijkman, Gaddah, Keshinro, Katwere, Douoguih, Robinson, Solforosi, Czapska-Casey, Dekking, Wollmann, Volkmann, Pau, Callendret, Sadoff, Schuitemaker, Zahn, Luhn, Hendriks and Roozendaal.
Conflict of interest statement
CM, KD, AG, BK, MK, MD, CR, LS, DC-C, LD, MP, BC, JS, HS, RZ, KL, JH, and RR are employees of Janssen Vaccines and Prevention, B.V., and may hold shares of Johnson & Johnson. YW and AV are employees of Bavarian Nordic and may hold shares in the company.
Figures

References
-
- Centers for Disease Control and Prevention . History of ebola virus disease (EVD) outbreaks (2023). Available at: https://www.cdc.gov/vhf/ebola/history/chronology.html (Accessed Accessed: 21 March 2023).
-
- Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, et al. . Safety and immunogenicity of novel adenovirus type 26-and modified vaccinia Ankara-vectored Ebola vaccines: A randomized clinical trial. JAMA - J Am Med Assoc (2016) 315(15):1610–23. doi: 10.1001/jama.2016.4218 - DOI - PubMed
-
- Anywaine Z, Whitworth H, Kaleebu P, Praygod G, Shukarev G, Manno D, et al. . Safety and immunogenicity of a 2-dose heterologous vaccination regimen with Ad26.ZEBOV and MVA-BN-filo ebola vaccines: 12-month data from a phase 1 randomized clinical trial in Uganda and Tanzania. J Infect Dis (2019) 220(1):46–56. doi: 10.1093/infdis/jiz070 - DOI - PMC - PubMed
-
- Mutua G, Anzala O, Luhn K, Robinson C, Bockstal V, Anumendem D, et al. . Safety and immunogenicity of a 2-dose heterologous vaccine regimen with ad26.ZEBOV and MVA-BN-filo ebola vaccines: 12-month data from a phase 1 randomized clinical trial in Nairobi, Kenya. J Infect Dis (2019) 220(1):57–67. doi: 10.1093/infdis/jiz071 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

