A real-world analysis of outcomes and healthcare costs of patients on perindopril/indapamide/amlodipine single-pill vs. multiple-pill combination in Italy
- PMID: 37728093
- PMCID: PMC10712996
- DOI: 10.1097/HJH.0000000000003570
A real-world analysis of outcomes and healthcare costs of patients on perindopril/indapamide/amlodipine single-pill vs. multiple-pill combination in Italy
Abstract
Objectives: This analysis compared adherence, cardiovascular (CV) events and all-cause mortality incidence, and healthcare costs among hypertensive patients treated with perindopril (PER)/indapamide (IND)/amlodipine (AML) in single-pill combination (SPC) vs. multiple-pill combination, in a real-world setting in Italy.
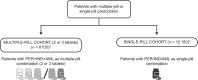
Methods: In this observational retrospective analysis of Italian administrative databases, adult patients treated with PER/IND/AML between 2010 and 2020 were divided into two cohorts: single-pill vs. multiple-pill. Patient data were available for at least one year before and after index date. Propensity score matching (PSM) was applied to reduce selection bias. Adherence was defined as proportion of days covered: non-adherence, <40%; partial adherence, 40-79%, and adherence ≥80%. Mortality incidence and CV events as single, or composite, endpoints were evaluated after first year of follow-up. Healthcare cost analyses were performed from the perspective of the Italian National Health Service.
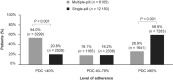
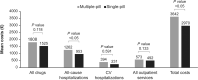
Results: Following PSM, the single-pill cohort included 12 150 patients, and the multiple-pill cohort, 6105. The SPC cohort had a significantly higher percentage of adherent patients vs. the multiple-pill cohort (59.9% vs. 26.9%, P < 0.001). Following the first year of follow-up, incidence of all-cause mortality, and combined endpoint of all-cause mortality and CV events were lower in the SPC cohort compared with multiple-pill cohort. Average annual direct healthcare costs were lower in the single-pill cohort (€2970) vs. multiple-pill cohort (€3642); cost of all drugs and all-cause hospitalizations were major contributors.
Conclusion: The SPC of PER/IND/AML, compared with multiple-pill combination, is associated with higher adherence to medication, lower incidence of CV events and mortality, and reduced healthcare costs.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
J.R.S. declares support for the present manuscript from Servier; grants or contracts from Servier; consulting fees from Servier; payment or honoraria from Servier; and support for attending meetings and/or travel from Servier. L.A.B. declares consulting fees from Servier; consulting fees from Medtronics to their institution; and payment or honoraria from Servier and Merck. P.B.J. declares support for the present manuscript from Servier; and consulting fees from Servier. A.K. declares support for the present manuscript from Servier; consulting fees from Servier; and payment or honoraria from Servier, Novartis, KRKA, and Shtada. L.D.E. and V.P. have no conflicts of interest. C.B. declares support for the present manuscript from Servier; consulting fees from Sanofi, Novo Nordisk, Alfasigma, Gilead, Recordati, Novartis, and Amarin; payment or honoraria from Servier, Menarini Asia-Pacific, Berlin Chemie, Novo Nordisk, and Gilead; and participation on a data safety monitoring board or advisory board for Alfasigma, Amarin, Boehringer Ingelheim, AstraZeneca, and Novartis.
Figures

References
-
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. . 2018 esc/esh guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–3104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

