Protective efficacy and correlates of immunity of immunodominant recombinant Babesia microti antigens
- PMID: 37728332
- PMCID: PMC10580920
- DOI: 10.1128/iai.00162-23
Protective efficacy and correlates of immunity of immunodominant recombinant Babesia microti antigens
Abstract
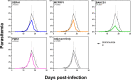
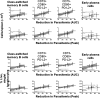
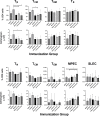
Babesia microti, an intraerythrocytic apicomplexan parasite, is the primary causative agent of human babesiosis and an emerging threat to public health in the United States and elsewhere. An effective vaccine against B. microti would reduce disease severity in acute babesiosis patients and shorten the parasitemic period in asymptomatic individuals, thereby minimizing the risk of transfusion-transmitted babesiosis. Here we report on immunogenicity, protective efficacy, and correlates of immunity following immunization with four immunodominant recombinantly produced B. microti antigens-Serine Reactive Antigen 1 (SERA1), Maltese Cross Form Related Protein 1 (MCFRP1), Piroplasm β-Strand Domain 1 (PiβS1), and Babesia microti Alpha Helical Cell Surface Protein 1 (BAHCS1)-delivered subcutaneously in Montanide ISA 51/CpG adjuvant in three doses to BALB/c mice. Following B. microti parasite challenge, BAHCS1 led to the highest reduction in peak parasitemia (67.8%), followed by SERA1 (44.8%) and MCFRP1 (41.9%); PiβS1 (27.6%) had minimal protective effect. All four B. microti antigens induced high ELISA total IgG and each isotype; however, antibody levels did not directly correlate with anti-parasitic activity in mice. Increased prechallenge levels of some cell populations including follicular helper T cells (TFH) and memory B cells, along with a set of six cytokines [IL-1α, IL-2, IL-3, IL-6, IL-12(p40), and G-CSF] that belong to both innate and adaptive immune responses, were generally associated with protective immunity. Our results indicate that mechanisms driving recombinant B. microti antigen-induced immunity are complex and multifactorial. We think that BAHCS1 warrants further evaluation in preclinical studies.
Keywords: Babesia microti; immune correlates; recombinant vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A Highly Sensitive Multiplex Antibody Assay Reduces Window Period for Detection of Babesia microti Infection.Open Forum Infect Dis. 2025 May 31;12(6):ofaf253. doi: 10.1093/ofid/ofaf253. eCollection 2025 Jun. Open Forum Infect Dis. 2025. PMID: 40453878 Free PMC article.
-
Babesia microti Protein BmSP44 Is a Novel Protective Antigen in a Mouse Model of Babesiosis.Front Immunol. 2020 Jul 7;11:1437. doi: 10.3389/fimmu.2020.01437. eCollection 2020. Front Immunol. 2020. PMID: 32733477 Free PMC article.
-
Antigen Discovery, Bioinformatics and Biological Characterization of Novel Immunodominant Babesia microti Antigens.Sci Rep. 2020 Jun 12;10(1):9598. doi: 10.1038/s41598-020-66273-6. Sci Rep. 2020. PMID: 32533024 Free PMC article.
-
Vaccination against babesiosis using recombinant GPI-anchored proteins.Int J Parasitol. 2019 Feb;49(2):175-181. doi: 10.1016/j.ijpara.2018.12.002. Epub 2019 Jan 24. Int J Parasitol. 2019. PMID: 30684517 Review.
-
Transfusion-transmitted Babesia spp.: bull's-eye on Babesia microti.Clin Microbiol Rev. 2011 Jan;24(1):14-28. doi: 10.1128/CMR.00022-10. Clin Microbiol Rev. 2011. PMID: 21233506 Free PMC article. Review.
Cited by
-
Identification of Babesia microti immunoreactive antigens by phage display cDNA screen.Infect Immun. 2024 Jul 11;92(7):e0021524. doi: 10.1128/iai.00215-24. Epub 2024 Jun 17. Infect Immun. 2024. PMID: 38884473 Free PMC article.
-
A Highly Sensitive Multiplex Antibody Assay Reduces Window Period for Detection of Babesia microti Infection.Open Forum Infect Dis. 2025 May 31;12(6):ofaf253. doi: 10.1093/ofid/ofaf253. eCollection 2025 Jun. Open Forum Infect Dis. 2025. PMID: 40453878 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

