Sensitivity of rapid antigen tests against SARS-CoV-2 Omicron and Delta variants
- PMID: 37728336
- PMCID: PMC10654096
- DOI: 10.1128/jcm.00138-23
Sensitivity of rapid antigen tests against SARS-CoV-2 Omicron and Delta variants
Abstract
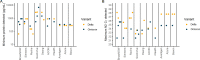
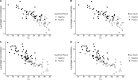
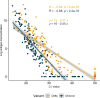
Rapid antigen tests (RATs) have become an invaluable tool for combating the COVID-19 pandemic. However, concerns have been raised regarding the ability of existing RATs to effectively detect emerging SARS-CoV-2 variants. We compared the performance of 10 commercially available, emergency use authorized RATs against the Delta and Omicron SARS-CoV-2 variants using both individual patient and serially diluted pooled clinical samples. The RATs exhibited lower sensitivity for Omicron samples when using PCR cycle threshold (CT) value (a rough proxy for RNA concentration) as the comparator. Interestingly, however, they exhibited similar sensitivity for Omicron and Delta samples when using quantitative antigen concentration as the comparator. We further found that the Omicron samples had lower ratios of antigen to RNA, which offers a potential explanation for the apparent lower sensitivity of RATs for that variant when using C T value as a reference. Our findings underscore the complexity in assessing RAT performance against emerging variants and highlight the need for ongoing evaluation in the face of changing population immunity and virus evolution.
Keywords: SARS-CoV-2; rapid antigen test.
Conflict of interest statement
A.L.G. reports contract testing from Abbott, Cepheid, Novavax, Pfizer, Janssen, and Hologic, and research support from Gilead and Merck, outside of the described work. The other authors have declared that no conflict of interest exists.
Figures
Update of
-
Sensitivity of Rapid Antigen Tests Against SARS-CoV-2 Omicron and Delta Variants.medRxiv [Preprint]. 2023 Feb 10:2023.02.09.23285583. doi: 10.1101/2023.02.09.23285583. medRxiv. 2023. Update in: J Clin Microbiol. 2023 Oct 24;61(10):e0013823. doi: 10.1128/jcm.00138-23. PMID: 36798414 Free PMC article. Updated. Preprint.
Similar articles
-
Sensitivity of Rapid Antigen Tests Against SARS-CoV-2 Omicron and Delta Variants.medRxiv [Preprint]. 2023 Feb 10:2023.02.09.23285583. doi: 10.1101/2023.02.09.23285583. medRxiv. 2023. Update in: J Clin Microbiol. 2023 Oct 24;61(10):e0013823. doi: 10.1128/jcm.00138-23. PMID: 36798414 Free PMC article. Updated. Preprint.
-
Evaluation of Four Point of Care (POC) Antigen Assays for the Detection of the SARS-CoV-2 Variant Omicron.Microbiol Spectr. 2022 Jun 29;10(3):e0102522. doi: 10.1128/spectrum.01025-22. Epub 2022 May 26. Microbiol Spectr. 2022. PMID: 35616382 Free PMC article.
-
Analysis of seven SARS-CoV-2 rapid antigen tests in detecting omicron (B.1.1.529) versus delta (B.1.617.2) using cell culture supernatants and clinical specimens.Infection. 2023 Feb;51(1):239-245. doi: 10.1007/s15010-022-01844-5. Epub 2022 May 20. Infection. 2023. PMID: 35596057 Free PMC article.
-
Revolutionizing SARS-CoV-2 omicron variant detection: Towards faster and more reliable methods.Talanta. 2024 Jan 1;266(Pt 1):124937. doi: 10.1016/j.talanta.2023.124937. Epub 2023 Jul 12. Talanta. 2024. PMID: 37481886 Review.
-
Molecular evolution of SARS-CoV-2 from December 2019 to August 2022.J Med Virol. 2023 Jan;95(1):e28366. doi: 10.1002/jmv.28366. J Med Virol. 2023. PMID: 36458547 Free PMC article. Review.
Cited by
-
The role of SARS-CoV-2 N protein in diagnosis and vaccination in the context of emerging variants: present status and prospects.Front Microbiol. 2023 Aug 14;14:1217567. doi: 10.3389/fmicb.2023.1217567. eCollection 2023. Front Microbiol. 2023. PMID: 37675423 Free PMC article. Review.
-
Key Insights into Respiratory Virus Testing: Sensitivity and Clinical Implications.Microorganisms. 2025 Jan 2;13(1):63. doi: 10.3390/microorganisms13010063. Microorganisms. 2025. PMID: 39858831 Free PMC article. Review.
-
Diagnostic Accuracy of the Abbott BinaxNOW COVID-19 Antigen Card Test, Puerto Rico.Influenza Other Respir Viruses. 2024 Jul;18(7):e13305. doi: 10.1111/irv.13305. Influenza Other Respir Viruses. 2024. PMID: 39053895 Free PMC article.
-
Detection of SARS-CoV-2 Using the Abbott™ PANBIO™ COVID-19 SELF-TEST Rapid Test in Patients Seen at INER.Biomedicines. 2025 Apr 22;13(5):1012. doi: 10.3390/biomedicines13051012. Biomedicines. 2025. PMID: 40426842 Free PMC article.
-
Designing and expression of novel recombinant fusion protein for efficient antigen screening of SARS-CoV-2.AMB Express. 2024 Jul 11;14(1):80. doi: 10.1186/s13568-024-01719-y. AMB Express. 2024. PMID: 38990364 Free PMC article.
References
-
- U.S. Food and Drug Administration . 2019. In vitro diagnostics EUAs - antigen diagnostic tests for SARS-CoV-2. Available from: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em...
-
- Osterman A, Badell I, Basara E, Stern M, Kriesel F, Eletreby M, Öztan GN, Huber M, Autenrieth H, Knabe R, Späth PM, Muenchhoff M, Graf A, Krebs S, Blum H, Durner J, Czibere L, Dächert C, Kaderali L, Baldauf H-M, Keppler OT. 2022. Impaired detection of Omicron by SARS-CoV-2 rapid antigen tests. Med Microbiol Immunol 211:105–117. doi:10.1007/s00430-022-00730-z - DOI - PMC - PubMed
-
- Schuit E, Venekamp RP, Veldhuijzen IK, van den Bijllaardt W, Pas SD, Stohr J, Lodder EB, Hellwich M, Molenkamp R, Igloi Z, Wijers C, Vroom IH, Nagel-Imming CRS, Han WGH, Kluytmans J, van den Hof S, van de Wijgert J, Moons KGM. 2022. Head-to-head comparison of the accuracy of saliva and nasal rapid antigen SARS-CoV-2 self-testing: cross-sectional study. BMC Med 20:406. doi:10.1186/s12916-022-02603-x - DOI - PMC - PubMed
-
- Eyre DW, Futschik M, Tunkel S, Wei J, Cole-Hamilton J, Saquib R, Germanacos N, Dodgson AR, Klapper PE, Sudhanva M, Kenny C, Marks P, Blandford E, Hopkins S, Peto TEA, Fowler T. 2023. Performance of antigen lateral flow devices in the UK during the alpha, Delta, and Omicron waves of the SARS-CoV-2 pandemic: a diagnostic and observational study. Lancet Infect Dis 23:922–932. doi:10.1016/S1473-3099(23)00129-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

