The morphogenic protein CopD controls the spatio-temporal dynamics of PBP1a and PBP2b in Streptococcus pneumoniae
- PMID: 37728370
- PMCID: PMC10653890
- DOI: 10.1128/mbio.01411-23
The morphogenic protein CopD controls the spatio-temporal dynamics of PBP1a and PBP2b in Streptococcus pneumoniae
Abstract
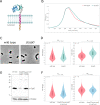
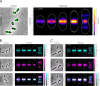
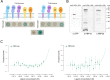
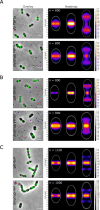
Penicillin-binding proteins (PBPs) are essential for proper bacterial cell division and morphogenesis. The genome of Streptococcus pneumoniae encodes for two class B PBPs (PBP2x and 2b), which are required for the assembly of the peptidoglycan framework and three class A PBPs (PBP1a, 1b and 2a), which remodel the peptidoglycan mesh during cell division. Therefore, their activities should be finely regulated in space and time to generate the pneumococcal ovoid cell shape. To date, two proteins, CozE and MacP, are known to regulate the function of PBP1a and PBP2a, respectively. In this study, we describe a novel regulator (CopD) that acts on both PBP1a and PBP2b. These findings provide valuable information for understanding bacterial cell division. Furthermore, knowing that ß-lactam antibiotic resistance often arises from PBP mutations, the characterization of such a regulator represents a promising opportunity to develop new strategies to resensitize resistant strains.
Keywords: Streptococcus pneumoniae; cell division; cell morphogenesis; penicillin-binding proteins; peptidoglycan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
