Removal of extracellular human amyloid beta aggregates by extracellular proteases in C. elegans
- PMID: 37728486
- PMCID: PMC10541181
- DOI: 10.7554/eLife.83465
Removal of extracellular human amyloid beta aggregates by extracellular proteases in C. elegans
Abstract
The amyloid beta (Aβ) plaques found in Alzheimer's disease (AD) patients' brains contain collagens and are embedded extracellularly. Several collagens have been proposed to influence Aβ aggregate formation, yet their role in clearance is unknown. To investigate the potential role of collagens in forming and clearance of extracellular aggregates in vivo, we created a transgenic Caenorhabditis elegans strain that expresses and secretes human Aβ1-42. This secreted Aβ forms aggregates in two distinct places within the extracellular matrix. In a screen for extracellular human Aβ aggregation regulators, we identified different collagens to ameliorate or potentiate Aβ aggregation. We show that a disintegrin and metalloprotease a disintegrin and metalloprotease 2 (ADM-2), an ortholog of ADAM9, reduces the load of extracellular Aβ aggregates. ADM-2 is required and sufficient to remove the extracellular Aβ aggregates. Thus, we provide in vivo evidence of collagens essential for aggregate formation and metalloprotease participating in extracellular Aβ aggregate removal.
Keywords: ADAM9; Alzheimer's disease; C. elegans; TIMP; aggregation; amyloid beta; cell biology; collagen; neuroscience.
© 2023, Jongsma et al.
Conflict of interest statement
EJ, AG, JM, CE No competing interests declared
Figures
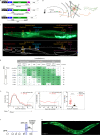
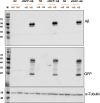
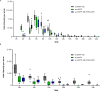



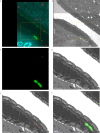


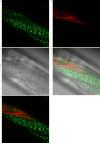
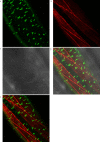

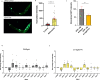

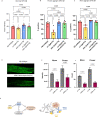
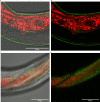
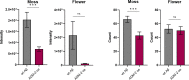


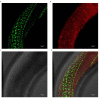
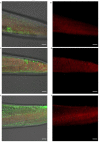





Update of
References
-
- Alzheimer’s Association Alzheimer’s Facts and Figures Report. 2022. [May 6, 2022]. https://www.alz.org/alzheimers-dementia/facts-figures
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

