Melatonin targets ferroptosis through bimodal alteration of redox environment and cellular pathways in NAFLD model
- PMID: 37728565
- PMCID: PMC10560965
- DOI: 10.1042/BSR20230128
Melatonin targets ferroptosis through bimodal alteration of redox environment and cellular pathways in NAFLD model
Abstract
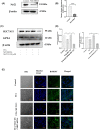
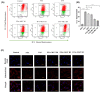
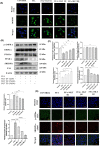
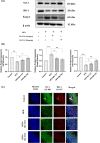
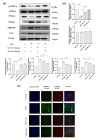
Ferroptosis is a non-conventional cellular death caused by lipid peroxide induced iron deposition. Intracellular lipid accumulation followed by generation of lipid peroxides is an hallmark of non-alcoholic fatty liver disease (NAFLD). Melatonin (MLT) is an important pineal hormone with tremendous antioxidant and anti-inflammatory properties. Various studies targeted ferroptosis in different diseases using melatonin. However, none of them focused the intrinsic mechanism of MLT's action to counteract ferroptosis in NAFLD. Hence, the present study investigated the role of MLT in improvement of NAFLD-induced ferroptosis. HepG2 cells were treated with free fatty acids (FFAs) to induce in vitro NAFLD state and C57BL/6 mice were fed with high-fat diet (HFD) followed by MLT administration. The results indicated that MLT administration caused the recovery from both FFA- and HFD-induced ferroptotic state via increasing GSH and SOD level, decreasing lipid reactive oxygen species (ROS) and malondialdehyde (MDA) level, increasing Nrf2 and HO-1 level to defend cells against an oxidative environment. MLT also altered the expression of two key proteins GPX4 and SLC7A11 back to their normal levels, which would otherwise cause ferroptosis. MLT also protected against histopathological damage of both liver tissue and HepG2 cells as depicted by Oil Red O, HE staining and immunofluorescence microscopy. MLT also had control over pAMPKα as well as PPARγ and PPARα responsible for lipid homeostasis and lipogenesis. In brief, MLT exerted its multifaceted effect in FFA- and HFD-induced NAFLD by retrieving cellular oxidative environment, reducing lipogenesis and lipid peroxidation and modulating Nrf2/HO-1 and GPX4/SLC7A11 axis to combat ferroptosis.
Keywords: Ferroptosis; Lipid peroxidation; Melatonin; NAFLD.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures





Similar articles
-
Melatonin suppresses atherosclerosis by ferroptosis inhibition via activating NRF2 pathway.FASEB J. 2024 May 31;38(10):e23678. doi: 10.1096/fj.202400427RR. FASEB J. 2024. PMID: 38780199
-
Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease.Toxicology. 2020 Dec 1;445:152599. doi: 10.1016/j.tox.2020.152599. Epub 2020 Sep 22. Toxicology. 2020. PMID: 32976958
-
[Tanshinone Ⅱ_A inhibits ferroptosis via Nrf2 signaling pathway to protect liver in rats of non-alcoholic fatty liver disease].Zhongguo Zhong Yao Za Zhi. 2024 Mar;49(6):1611-1620. doi: 10.19540/j.cnki.cjcmm.20231229.501. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 38621946 Chinese.
-
Lipid Peroxidation in Ferroptosis and Association with Nonalcoholic Fatty Liver Disease.Front Biosci (Landmark Ed). 2023 Dec 6;28(12):332. doi: 10.31083/j.fbl2812332. Front Biosci (Landmark Ed). 2023. PMID: 38179745 Review.
-
Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis.Int J Mol Sci. 2022 Dec 27;24(1):449. doi: 10.3390/ijms24010449. Int J Mol Sci. 2022. PMID: 36613888 Free PMC article. Review.
Cited by
-
Alterations in Glutathione Redox Homeostasis in Metabolic Dysfunction-Associated Fatty Liver Disease: A Systematic Review.Antioxidants (Basel). 2024 Nov 28;13(12):1461. doi: 10.3390/antiox13121461. Antioxidants (Basel). 2024. PMID: 39765791 Free PMC article. Review.
-
Killing hepatocellular carcinoma in the NAFLD/NASH stage: a comprehensive perspective on targeting regulated cell death.Cell Death Discov. 2025 Jun 19;11(1):281. doi: 10.1038/s41420-025-02558-x. Cell Death Discov. 2025. PMID: 40537476 Free PMC article. Review.
-
Melatonin Prevents the Progression of MASLD via Inhibiting FFAs-Induced Ferroptosis through KEAP1/NRF2/HO-1 Pathway.Biomol Ther (Seoul). 2025 Sep 1;33(5):876-889. doi: 10.4062/biomolther.2025.037. Epub 2025 Aug 18. Biomol Ther (Seoul). 2025. PMID: 40820540 Free PMC article.
-
Targeting cell death in NAFLD: mechanisms and targeted therapies.Cell Death Discov. 2024 Sep 7;10(1):399. doi: 10.1038/s41420-024-02168-z. Cell Death Discov. 2024. PMID: 39244571 Free PMC article. Review.
-
Investigation of Melatonin Incorporated CMC-Gelatin Based Edible Coating on the Alleviation of Chilling Injury Induced Pericarp Browning in Longkong.Foods. 2023 Dec 24;13(1):72. doi: 10.3390/foods13010072. Foods. 2023. PMID: 38201100 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

