Perspectives on Osteoarthritis Treatment with Mesenchymal Stem Cells and Radix Achyranthis Bidentatae
- PMID: 37728585
- PMCID: PMC11081162
- DOI: 10.14336/AD.2023.0817
Perspectives on Osteoarthritis Treatment with Mesenchymal Stem Cells and Radix Achyranthis Bidentatae
Abstract
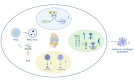
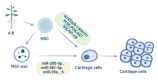
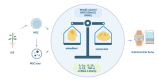
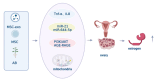
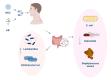
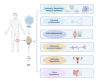
Knee osteoarthritis, a widespread degenerative condition, impacts a younger population and leads to high disability rates. Nature often provides solutions for aging and disease prevention. Mesenchymal stem cells (MSCs) and Radix Achyranthis Bidentatae (AB) are natural substances with potential. MSCs can transform into various tissues, alleviating symptoms by releasing factors and miRNA, potentially slowing osteoarthritis progression. AB's compositions target knee joint cells, enhancing internal conditions and joint function. Both MSCs and AB share mechanisms for immune regulation, reducing cartilage apoptosis, promoting chondrocyte formation, and addressing osteoporosis. They also influence estrogen and gut flora. This article reviews their roles in treating osteoarthritis, discussing apoptosis reduction, chondrocyte growth, bone enhancement, angiogenesis, and regulation of estrogen and intestinal flora. It explores their relationship and suggests AB's potential in stimulating mesenchymal stem cell repair for knee osteoarthritis treatment.
Figures
Similar articles
-
Integrated Network Pharmacology and Experimental Validation Approach to Investigate the Mechanisms of Radix Rehmanniae Praeparata - Angelica Sinensis - Radix Achyranthis Bidentatae in Treating Knee Osteoarthritis.Drug Des Devel Ther. 2024 May 15;18:1583-1602. doi: 10.2147/DDDT.S455006. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38765877 Free PMC article.
-
Network Pharmacology Approach to Uncover the Mechanism Governing the Effect of Radix Achyranthis Bidentatae on Osteoarthritis.BMC Complement Med Ther. 2020 Apr 21;20(1):121. doi: 10.1186/s12906-020-02909-4. BMC Complement Med Ther. 2020. PMID: 32316966 Free PMC article.
-
Prediction of the molecular mechanism of eucommiae Cortex - Achyranthis bidentatae radix in the treatment of osteoarthritis: network pharmacology and molecular docking.Drug Dev Ind Pharm. 2021 Aug;47(8):1235-1247. doi: 10.1080/03639045.2021.1988098. Epub 2021 Dec 2. Drug Dev Ind Pharm. 2021. PMID: 34590537
-
Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence.Int J Mol Sci. 2022 Dec 21;24(1):59. doi: 10.3390/ijms24010059. Int J Mol Sci. 2022. PMID: 36613502 Free PMC article. Review.
-
Harnessing knee joint resident mesenchymal stem cells in cartilage tissue engineering.Acta Biomater. 2023 Sep 15;168:372-387. doi: 10.1016/j.actbio.2023.07.024. Epub 2023 Jul 21. Acta Biomater. 2023. PMID: 37481194 Review.
Cited by
-
Mechanisms and challenges of mesenchymal stem cells in the treatment of knee osteoarthritis.World J Stem Cells. 2025 Apr 26;17(4):102923. doi: 10.4252/wjsc.v17.i4.102923. World J Stem Cells. 2025. PMID: 40308880 Free PMC article.
-
Effectiveness and safety of Chinese herbal footbaths as an adjuvant therapy for dysmenorrhea: a systematic review and meta-analysis.Front Pharmacol. 2024 Aug 5;15:1397359. doi: 10.3389/fphar.2024.1397359. eCollection 2024. Front Pharmacol. 2024. PMID: 39161905 Free PMC article.
-
Advancing Neurological Health: Insights into Aging, Immunity, and Vascular Dynamics.Aging Dis. 2024 May 7;15(3):939-944. doi: 10.14336/AD.2024.0423. Aging Dis. 2024. PMID: 38722789 Free PMC article.
-
Emerging Therapy in Osteoarthritis: Mesenchymal Stem Cells, Secretomes, and Using Hydrogels to Enhance Efficacy.BioDrugs. 2025 Jul;39(4):555-571. doi: 10.1007/s40259-025-00728-y. Epub 2025 May 30. BioDrugs. 2025. PMID: 40447967 Review.
-
Cell-free osteoarthritis treatment with dual-engineered chondrocyte-targeted extracellular vesicles derived from mechanical loading primed mesenchymal stem cells.J Tissue Eng. 2025 Feb 8;16:20417314241312563. doi: 10.1177/20417314241312563. eCollection 2025 Jan-Dec. J Tissue Eng. 2025. PMID: 39926048 Free PMC article.
References
-
- Felson DT, Zhang Y (1998). An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheumatol, 41:1343-1355. - PubMed
-
- Radin E, Paul I, Rose R (1972). Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet, 7749:519-522. - PubMed
-
- Chiou AF, Buschmann MT (1999). The factors associated with excess disability in arthritic elderly patients. J Ment Health Aging, 5:151-164
-
- Liu X, Qu X, Chen Y, Liao L, Cheng K, Shao C, et al.. (2012). Mesenchymal stem/stromal cells induce the generation of novel IL-10-dependent regulatory dendritic cells by SOCS3 activation. J Immunol, 189:1182-1192. - PubMed
-
- Zhang B, Liu R, Shi D, Liu X, Chen Y, Dou X, et al.. (2009). Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2 dependent regulatory dendritic cell population. Blood, 113:46-57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
