Single molecule analysis of CENP-A chromatin by high-speed atomic force microscopy
- PMID: 37728600
- PMCID: PMC10511241
- DOI: 10.7554/eLife.86709
Single molecule analysis of CENP-A chromatin by high-speed atomic force microscopy
Abstract
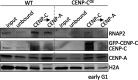
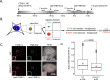
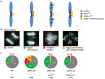
Chromatin accessibility is modulated in a variety of ways to create open and closed chromatin states, both of which are critical for eukaryotic gene regulation. At the single molecule level, how accessibility is regulated of the chromatin fiber composed of canonical or variant nucleosomes is a fundamental question in the field. Here, we developed a single-molecule tracking method where we could analyze thousands of canonical H3 and centromeric variant nucleosomes imaged by high-speed atomic force microscopy. This approach allowed us to investigate how changes in nucleosome dynamics in vitro inform us about transcriptional potential in vivo. By high-speed atomic force microscopy, we tracked chromatin dynamics in real time and determined the mean square displacement and diffusion constant for the variant centromeric CENP-A nucleosome. Furthermore, we found that an essential kinetochore protein CENP-C reduces the diffusion constant and mobility of centromeric nucleosomes along the chromatin fiber. We subsequently interrogated how CENP-C modulates CENP-A chromatin dynamics in vivo. Overexpressing CENP-C resulted in reduced centromeric transcription and impaired loading of new CENP-A molecules. From these data, we speculate that factors altering nucleosome mobility in vitro, also correspondingly alter transcription in vivo. Subsequently, we propose a model in which variant nucleosomes encode their own diffusion kinetics and mobility, and where binding partners can suppress or enhance nucleosome mobility.
Keywords: chromatin; chromosomes; epigenetics; gene expression; high-speed AFM; human; none; nucleosomes; single-molecule.
Conflict of interest statement
DM, KN, RB, TR No competing interests declared, YD Reviewing editor, eLife
Figures











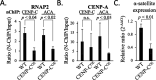



Update of
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

