Analysis of image data from the EuroNet PHL-C2 trial indicates a potential reduction in injected F-18 FDG activities in children: a proposal to update the EANM Paediatric Dosage Card
- PMID: 37728668
- PMCID: PMC10774179
- DOI: 10.1007/s00259-023-06396-w
Analysis of image data from the EuroNet PHL-C2 trial indicates a potential reduction in injected F-18 FDG activities in children: a proposal to update the EANM Paediatric Dosage Card
Abstract
Background: The aim of this work is to provide the currently missing evidence that may allow an update of the Paediatric Dosage Card provided by the European Association of Nuclear Medicine (EANM) for conventional PET/CT systems.
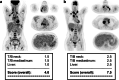
Methods: In a total of 2082 consecutive [18F]FDG-PET scans performed within the EuroNet-PHL-C2 trial, the administered [18F]FDG activity was compared to the activity recommended by the EANM Paediatric Dosage Card. None of these scans had been rejected beforehand by the reference nuclear medicine panel of the trial because of poor image quality. For detailed quality assessment, a subset of 91 [18F]FDG-PET scans, all performed in different patients at staging, was selected according to pre-defined criteria, which (a) included only patients who had received substantially lower activities than those recommended by the EANM Paediatric Dosage Card, and (b) included as wide a range of different PET systems and imaging parameters as possible to ensure that the conclusions drawn in this work are as generally valid as possible. The image quality of the subset was evaluated visually by two independent readers using a quality scoring system as well as analytically based on a volume-of-interest analysis in 244 lesions and the healthy liver. Finally, recommendations for an update of the EANM Paediatric Dosage Card were derived based on the available data.
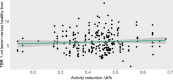
Results: The activity recommended by the EANM Paediatric Dosage Card was undercut by a median of 99.4 MBq in 1960 [18F]FDG-PET scans and exceeded by a median of 15.1 MBq in 119 scans. In the subset analysis (n = 91), all image data were visually classified as clinically useful. In addition, only a very weak correlation (r = 0.06) between activity reduction and tumour-to-background ratio was found. Due to the intended heterogeneity of the dataset, the noise could not be analysed statistically sound as the high range of different imaging variables resulted in very small subsets. Finally, a suggestion for an update of the EANM Paediatric Dosage Card was developed, based on the analysis presented, resulting in a mean activity reduction by 39%.
Conclusion: The results of this work allow for a conservative update of the EANM Paediatric Dosage Card for [18F]FDG-PET/CT scans performed with conventional PET/CT systems.
Keywords: Activity reduction; EANM Paediatric Dosage Card; EuroNet-PHL-C2 trial; New activity recommendation for [18F]FDG-PET; Radiation protection; [18F]FDG dosage; [18F]FDG-PET.
© 2023. The Author(s).
Conflict of interest statement
M Lassmann has received institutional grants by IPSEN Pharma, Nordic Nanovector and Novartis. No other potential conflicts of interest relevant to this article exist.
Figures


Similar articles
-
The effect of modern PET technology and techniques on the EANM paediatric dosage card.Eur J Nucl Med Mol Imaging. 2022 May;49(6):1964-1969. doi: 10.1007/s00259-021-05635-2. Epub 2021 Dec 15. Eur J Nucl Med Mol Imaging. 2022. PMID: 34910233 Free PMC article.
-
An Australian local diagnostic reference level for paediatric whole-body 18F-FDG PET/CT.Br J Radiol. 2019 Apr;92(1096):20180879. doi: 10.1259/bjr.20180879. Epub 2019 Mar 5. Br J Radiol. 2019. PMID: 30653334 Free PMC article.
-
The new EANM paediatric dosage card: additional notes with respect to F-18.Eur J Nucl Med Mol Imaging. 2008 Sep;35(9):1666-8. doi: 10.1007/s00259-008-0799-9. Epub 2008 Jun 24. Eur J Nucl Med Mol Imaging. 2008. PMID: 18574583
-
Role of [18F]FDG PET/CT in patients with invasive breast carcinoma of no special type: Literature review and comparison between guidelines.Breast. 2024 Dec;78:103806. doi: 10.1016/j.breast.2024.103806. Epub 2024 Sep 12. Breast. 2024. PMID: 39303572 Free PMC article. Review.
-
Perspectives on joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards for [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors.Cancer Imaging. 2022 Dec 20;22(1):73. doi: 10.1186/s40644-022-00512-z. Cancer Imaging. 2022. PMID: 36539908 Free PMC article. Review.
Cited by
-
A low-dose protocol in pediatric 18F-FDG scans using 30-cm axis field of view PET/CT.Ann Nucl Med. 2025 Jun;39(6):546-551. doi: 10.1007/s12149-025-02030-x. Epub 2025 Feb 28. Ann Nucl Med. 2025. PMID: 40019733
References
-
- Lassmann M, Treves ST. Pediatric radiopharmaceutical administration: harmonization of the 2007 EANM Paediatric Dosage Card (version 1.5.2008) and the 2010 North American Consensus guideline. Eur J Nucl Med Mol Imaging. 2014;41:1636. 10.1007/s00259-014-2731-9. - PubMed
-
- Treves ST, Gelfand MJ, Fahey FH, Parisi MT. 2016 update of the North American consensus guidelines for pediatric administered radiopharmaceutical activities. J Nucl Med. 2016;57:15N–N18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

