Human CD34+-derived complete plasmacytoid and conventional dendritic cell vaccine effectively induces antigen-specific CD8+ T cell and NK cell responses in vitro and in vivo
- PMID: 37728691
- PMCID: PMC10511603
- DOI: 10.1007/s00018-023-04923-4
Human CD34+-derived complete plasmacytoid and conventional dendritic cell vaccine effectively induces antigen-specific CD8+ T cell and NK cell responses in vitro and in vivo
Abstract
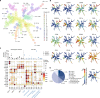
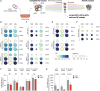
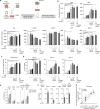
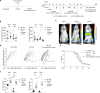
Allogeneic stem cell transplantation (alloSCT) can be curative for hemato-oncology patients due to effective graft-versus-tumor immunity. However, relapse remains the major cause of treatment failure, emphasizing the need for adjuvant immunotherapies. In this regard, post-transplantation dendritic cell (DC) vaccination is a highly interesting strategy to boost graft-versus-tumor responses. Previously, we developed a clinically applicable protocol for simultaneous large-scale generation of end-stage blood DC subsets from donor-derived CD34+ stem cells, including conventional type 1 and 2 DCs (cDC1s and cDC2s), and plasmacytoid DCs (pDCs). In addition, the total cultured end-product (DC-complete vaccine), also contains non-end-stage-DCs (i.e. non-DCs). In this study, we aimed to dissect the phenotypic identity of these non-DCs and their potential immune modulatory functions on the potency of cDCs and pDCs in stimulating tumor-reactive CD8+ T and NK cell responses, in order to obtain rationale for clinical translation of our DC-complete vaccine. The non-DC compartment was heterogeneous and comprised of myeloid progenitors and (immature) granulocyte- and monocyte-like cells. Importantly, non-DCs potentiated toll-like receptor-induced DC maturation, as reflected by increased expression of co-stimulatory molecules and enhanced cDC-derived IL-12 and pDC-derived IFN-α production. Additionally, antigen-specific CD8+ T cells effectively expanded upon DC-complete vaccination in vitro and in vivo. This effect was strongly augmented by non-DCs in an antigen-independent manner. Moreover, non-DCs did not impair in vitro DC-mediated NK cell activation, degranulation nor cytotoxicity. Notably, in vivo i.p. DC-complete vaccination activated i.v. injected NK cells. Together, these data demonstrate that the non-DC compartment potentiates DC-mediated activation and expansion of antigen-specific CD8+ T cells and do not impair NK cell responses in vitro and in vivo. This underscores the rationale for further clinical translation of our CD34+-derived DC-complete vaccine in hemato-oncology patients post alloSCT.
Keywords: CD34+ progenitor cells; Dendritic cells; Immunotherapy; NK cells; T cells; Vaccination.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Clinically applicable CD34+-derived blood dendritic cell subsets exhibit key subset-specific features and potently boost anti-tumor T and NK cell responses.Cancer Immunol Immunother. 2021 Nov;70(11):3167-3181. doi: 10.1007/s00262-021-02899-3. Epub 2021 Apr 1. Cancer Immunol Immunother. 2021. PMID: 33796917 Free PMC article.
-
Hematopoietic stem cell-derived myeloid and plasmacytoid DC-based vaccines are highly potent inducers of tumor-reactive T cell and NK cell responses ex vivo.Oncoimmunology. 2017 Feb 6;6(3):e1285991. doi: 10.1080/2162402X.2017.1285991. eCollection 2017. Oncoimmunology. 2017. PMID: 28405517 Free PMC article.
-
CD34-derived dendritic cells transfected ex vivo with HIV-Gag mRNA induce polyfunctional T-cell responses in nonhuman primates.Eur J Immunol. 2012 Aug;42(8):2019-30. doi: 10.1002/eji.201242478. Epub 2012 Jul 4. Eur J Immunol. 2012. PMID: 22585548 Free PMC article.
-
DC-Based Vaccines for Cancer Immunotherapy.Vaccines (Basel). 2020 Nov 26;8(4):706. doi: 10.3390/vaccines8040706. Vaccines (Basel). 2020. PMID: 33255895 Free PMC article. Review.
-
Plasmacytoid Dendritic Cells as Cell-Based Therapeutics: A Novel Immunotherapy to Treat Human Immunodeficiency Virus Infection?Front Cell Infect Microbiol. 2020 May 26;10:249. doi: 10.3389/fcimb.2020.00249. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32528903 Free PMC article. Review.
Cited by
-
cGAS-STING pathway mediates activation of dendritic cell sensing of immunogenic tumors.Cell Mol Life Sci. 2024 Mar 21;81(1):149. doi: 10.1007/s00018-024-05191-6. Cell Mol Life Sci. 2024. PMID: 38512518 Free PMC article. Review.
-
Human CD34+-derived plasmacytoid dendritic cells as surrogates for primary pDCs and potential cancer immunotherapy.Front Immunol. 2024 Nov 7;15:1433119. doi: 10.3389/fimmu.2024.1433119. eCollection 2024. Front Immunol. 2024. PMID: 39575246 Free PMC article.
References
-
- de Lima M, et al. Proceedings from the National Cancer Institute's Second International Workshop on the Biology, Prevention, and Treatment of Relapse After Hematopoietic Stem Cell Transplantation: part III. Prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transpl. 2014;20(1):4–13. doi: 10.1016/j.bbmt.2013.08.012. - DOI - PMC - PubMed
-
- Avigan D, et al. Proceedings from the National Cancer Institute's Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: part II. Autologous Transplantation-novel agents and immunomodulatory strategies. Biol Blood Marrow Transpl. 2013;19(12):1661–1669. doi: 10.1016/j.bbmt.2013.08.011. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

