Human CD34+-derived complete plasmacytoid and conventional dendritic cell vaccine effectively induces antigen-specific CD8+ T cell and NK cell responses in vitro and in vivo
- PMID: 37728691
- PMCID: PMC10511603
- DOI: 10.1007/s00018-023-04923-4
Human CD34+-derived complete plasmacytoid and conventional dendritic cell vaccine effectively induces antigen-specific CD8+ T cell and NK cell responses in vitro and in vivo
Abstract
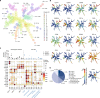
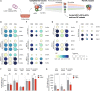
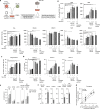
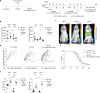
Allogeneic stem cell transplantation (alloSCT) can be curative for hemato-oncology patients due to effective graft-versus-tumor immunity. However, relapse remains the major cause of treatment failure, emphasizing the need for adjuvant immunotherapies. In this regard, post-transplantation dendritic cell (DC) vaccination is a highly interesting strategy to boost graft-versus-tumor responses. Previously, we developed a clinically applicable protocol for simultaneous large-scale generation of end-stage blood DC subsets from donor-derived CD34+ stem cells, including conventional type 1 and 2 DCs (cDC1s and cDC2s), and plasmacytoid DCs (pDCs). In addition, the total cultured end-product (DC-complete vaccine), also contains non-end-stage-DCs (i.e. non-DCs). In this study, we aimed to dissect the phenotypic identity of these non-DCs and their potential immune modulatory functions on the potency of cDCs and pDCs in stimulating tumor-reactive CD8+ T and NK cell responses, in order to obtain rationale for clinical translation of our DC-complete vaccine. The non-DC compartment was heterogeneous and comprised of myeloid progenitors and (immature) granulocyte- and monocyte-like cells. Importantly, non-DCs potentiated toll-like receptor-induced DC maturation, as reflected by increased expression of co-stimulatory molecules and enhanced cDC-derived IL-12 and pDC-derived IFN-α production. Additionally, antigen-specific CD8+ T cells effectively expanded upon DC-complete vaccination in vitro and in vivo. This effect was strongly augmented by non-DCs in an antigen-independent manner. Moreover, non-DCs did not impair in vitro DC-mediated NK cell activation, degranulation nor cytotoxicity. Notably, in vivo i.p. DC-complete vaccination activated i.v. injected NK cells. Together, these data demonstrate that the non-DC compartment potentiates DC-mediated activation and expansion of antigen-specific CD8+ T cells and do not impair NK cell responses in vitro and in vivo. This underscores the rationale for further clinical translation of our CD34+-derived DC-complete vaccine in hemato-oncology patients post alloSCT.
Keywords: CD34+ progenitor cells; Dendritic cells; Immunotherapy; NK cells; T cells; Vaccination.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- de Lima M, et al. Proceedings from the National Cancer Institute's Second International Workshop on the Biology, Prevention, and Treatment of Relapse After Hematopoietic Stem Cell Transplantation: part III. Prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transpl. 2014;20(1):4–13. doi: 10.1016/j.bbmt.2013.08.012. - DOI - PMC - PubMed
-
- Avigan D, et al. Proceedings from the National Cancer Institute's Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: part II. Autologous Transplantation-novel agents and immunomodulatory strategies. Biol Blood Marrow Transpl. 2013;19(12):1661–1669. doi: 10.1016/j.bbmt.2013.08.011. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

