A Real-World Effectiveness Study Using a Mobile Application to Evaluate Early Outcomes with Upadacitinib in Rheumatoid Arthritis
- PMID: 37728861
- PMCID: PMC10654297
- DOI: 10.1007/s40744-023-00594-6
A Real-World Effectiveness Study Using a Mobile Application to Evaluate Early Outcomes with Upadacitinib in Rheumatoid Arthritis
Abstract
Introduction: The impact of upadacitinib on rheumatoid arthritis (RA) symptoms was evaluated during the first 12 weeks of treatment via patient-reported outcomes (PROs) using a mobile health application (app).
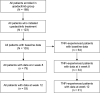
Methods: Participating rheumatologists from the CorEvitas RA Registry (prospective, observational cohort) recruited patients with RA initiating upadacitinib treatment. A modified version of the ArthritisPower® app was used to collect PROs, including the Routine Assessment of Patient Index Data 3 (RAPID3), duration of morning joint stiffness, and the Patient-Reported Outcomes Measurement Information System (PROMIS)-Fatigue 7a Short Form at baseline and weeks 1-4, 8, and 12. RAPID3 responses over time were assessed using Kaplan-Meier estimation to determine the proportion of patients achieving disease activity improvement and minimal clinically important difference (MCID). Results were analyzed for all patients initiating upadacitinib and a subsample of TNF inhibitor (TNFi)-experienced patients with moderate to severe disease at baseline.
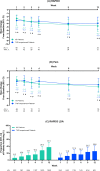
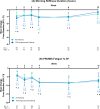
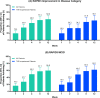
Results: A total of 103 patients with RA initiating upadacitinib (62.1% TNFi-experienced) were included. At week 12, 53 patients (51.4%) completed the study and provided PRO data via the app. Among all patients, improvements in RAPID3, pain, morning stiffness, and fatigue were observed at week 1 and were maintained or further improved through week 12. At week 12, 37.5% of patients achieved RAPID3 low disease activity. Starting at week 1, improvements in RAPID3 disease activity category (19.4% of patients) and achievement of MCID (16.3%) were reported, with nearly 50% of patients achieving these outcomes by week 4 (RAPID3 category: 48.8%; MCID: 49.2%) and 60% by week 12 (RAPID3 category: 59.6%; MCID: 59.8%). TNFi-experienced patients generally reported similar outcomes. Patient-reported medication convenience and compliance were generally high.
Conclusions: In this real-world cohort of patients with RA, treatment with upadacitinib was associated with early and significant improvement in RAPID3, pain, morning stiffness, and fatigue regardless of prior TNFi experience. Clinically meaningful improvement in RAPID3 patient-reported disease activity was observed as early as week 1, with continued improvement reported through week 12.
Keywords: CorEvitas; Digital health; Janus kinase (JAK) inhibitor; Observational; Patient-reported outcomes; Prospective; Real-world effectiveness; Rheumatoid arthritis; Routine Assessment of Patient Index Data 3 (RAPID3); Upadacitinib.
© 2023. The Author(s).
Conflict of interest statement
Financial arrangements of the authors with companies whose products may be related to the present manuscript are listed, as declared by the authors. Leslie R. Harrold: Employee and shareholder of CorEvitas, consultant to AbbVie, Bristol Myers Squibb, and Roche, speaker’s bureau for Bristol Myers Squibb. Patrick Zueger: Employee of AbbVie and may hold stock or stock options. W. Benjamin Nowell: Employee of Global Healthy Living Foundation, an independent nonprofit research organization, principal investigator for studies with grant support from AbbVie, Amgen, Janssen, and Scipher Medicine. Taylor Blachley: Former employee of CorEvitas; current employee of Syneos Health. Amy Schrader: Employee of CorEvitas. Paul R. Lakin: Employee of CorEvitas. David Curtis: Employee of Global Healthy Living Foundation, an independent nonprofit research organization. Laura Stradford: Employee of Global Healthy Living Foundation, an independent nonprofit research organization. Shilpa Venkatachalam: Employee of Global Healthy Living Foundation, an independent nonprofit research organization. Namita Tundia: Former employee of AbbVie and may hold stock or stock options; current employee of EMD Serono. Pankaj A. Patel: Employee of AbbVie and may hold stock or stock options.
Figures
Similar articles
-
Routine Assessment of Patient Index Data 3 (RAPID3) in Patients with Rheumatoid Arthritis Treated with Long-Term Upadacitinib Therapy in Five Randomized Controlled Trials.Rheumatol Ther. 2022 Dec;9(6):1517-1529. doi: 10.1007/s40744-022-00483-4. Epub 2022 Sep 20. Rheumatol Ther. 2022. PMID: 36125701 Free PMC article.
-
Real-World Use and Effectiveness Outcomes in Patients with Rheumatoid Arthritis Treated with Upadacitinib: An Analysis from the CorEvitas Registry.Rheumatol Ther. 2024 Apr;11(2):363-380. doi: 10.1007/s40744-024-00639-4. Epub 2024 Feb 12. Rheumatol Ther. 2024. PMID: 38345715 Free PMC article.
-
Effects of upadacitinib on patient-reported outcomes: results from SELECT-BEYOND, a phase 3 randomized trial in patients with rheumatoid arthritis and inadequate responses to biologic disease-modifying antirheumatic drugs.Arthritis Res Ther. 2019 Dec 2;21(1):263. doi: 10.1186/s13075-019-2059-8. Arthritis Res Ther. 2019. PMID: 31791386 Free PMC article. Clinical Trial.
-
RAPID3, an index to assess and monitor patients with rheumatoid arthritis, without formal joint counts: similar results to DAS28 and CDAI in clinical trials and clinical care.Rheum Dis Clin North Am. 2009 Nov;35(4):773-8, viii. doi: 10.1016/j.rdc.2009.10.008. Rheum Dis Clin North Am. 2009. PMID: 19962621 Review.
-
Can RAPID3, an index without formal joint counts or laboratory tests, serve to guide rheumatologists in tight control of rheumatoid arthritis in usual clinical care?Bull NYU Hosp Jt Dis. 2009;67(3):254-66. Bull NYU Hosp Jt Dis. 2009. PMID: 19852747 Review.
Cited by
-
Achievement of treatment targets and maintenance of response with upadacitinib in patients with moderate-to-severe rheumatoid arthritis in real-world practice: 1-year outcomes from the UPHOLD observational study.Arthritis Res Ther. 2025 Apr 10;27(1):84. doi: 10.1186/s13075-025-03528-5. Arthritis Res Ther. 2025. PMID: 40211417 Free PMC article.
-
Exploring the Potential of Electronic Patient-Generated Health Data for Evaluating Treatment Response to Intramuscular Steroids in Rheumatoid Arthritis: Case Series.JMIR Form Res. 2024 Oct 28;8:e55715. doi: 10.2196/55715. JMIR Form Res. 2024. PMID: 39467551 Free PMC article.
References
-
- Harrold L, Zueger P, Nowell WB, Blachley T, Lakin P, Curtis D, et al. Early real-world effectiveness of upadacitinib in rheumatoid arthritis using patient-reported outcomes collected via mobile application [abstract]. Arthritis Rheumatol. 2022;74(suppl 9):2656–58.
LinkOut - more resources
Full Text Sources
Miscellaneous

