Contribution of serology in congenital toxoplasmosis diagnosis: results from a 10-year French retrospective study
- PMID: 37728898
- PMCID: PMC10595068
- DOI: 10.1128/jcm.00354-23
Contribution of serology in congenital toxoplasmosis diagnosis: results from a 10-year French retrospective study
Abstract
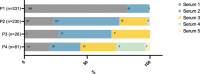
This study aimed to evaluate different serological strategies for the postnatal diagnosis of congenital toxoplasmosis (CT) and establish a biological algorithm for CT diagnosis. The study analyzed serological data of immunoglobulins M, A, and G (IgM, IgA, IgG) performed by immunoenzymatic and compared immunological profile (CIP) assays in 668 newborns with CT diagnosis across four testing periods: P1 (D0- D10), P2 (D11-D35), P3 (D36-D45), and P4 (>D45). Forty-nine percent of the 668 CT cases were diagnosed during P1 and 34%, 4%, and 12% during P2, P3, and P4, respectively. CIP assays detected neosynthetized IgMs/IgGs in 98% of CT cases diagnosed during P1, while IgMs and IgAs were detected in 90% and 57% of CT cases diagnosed during P2 and in 88% and 67% of diagnoses made during P3, respectively. Detection of neosynthesized IgMs/IgGs, IgMs, and IgAs by immunoassay contributed to CT diagnosis in 81%, 77%, and 60% of cases, respectively. In total, 46% of serum samples were positive for all three parameters, 27% for two, and 27% for one of the three. The study recommends using the CIP assay as standard during P1 for CT diagnosis and IgM and IgA immunoassays after P1. A clinical and biological follow-up in a specialized center with a close collaboration between biologists and clinicians is highly recommended to increase the chances of early diagnosis. Overall, this study provides useful information for the development of a biological algorithm for CT diagnosis, which can aid in early detection and appropriate treatment of this disease.
Keywords: CIP assay; IgA; IgM; congenital toxoplasmosis; immunoblot; serological diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- HAS . 2017. Diagnostic Biologique de la Toxoplasmose Acquise Du Sujet Immunocompétent (doNT La femme Enceinte), La Toxoplasmose Congénitale (diagnostic Pré- et postnatal) et La Toxoplasmose Oculaire
-
- Peyron F, L’ollivier C, Mandelbrot L, Wallon M, Piarroux R, Kieffer F, Hadjadj E, Paris L, Garcia-Meric P. 2019. Maternal and congenital toxoplasmosis: diagnosis and treatment recommendations of a French multidisciplinary working group. Pathogens 8:24. doi:10.3390/pathogens8010024 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

