Circulating Tumor DNA Dynamics as Prognostic Markers in Locally Advanced and Metastatic Esophageal Squamous Cell Carcinoma
- PMID: 37728901
- PMCID: PMC10512170
- DOI: 10.1001/jamasurg.2023.4395
Circulating Tumor DNA Dynamics as Prognostic Markers in Locally Advanced and Metastatic Esophageal Squamous Cell Carcinoma
Abstract
Importance: Esophageal squamous cell carcinoma (ESCC) is a deadly disease with frequent recurrence. There are unmet needs for prognostic biomarkers for dynamically monitoring disease progression and detecting minimal residual disease.
Objective: To examine whether circulating tumor DNA is clinically useful as a prognostic biomarker for ESCC recurrence and patient survival.
Design, setting, and participants: This single-center, population-based cohort study consecutively enrolled 147 patients receiving curative (n = 74) or palliative (n = 73) treatment at the surgery and clinical oncology departments of Queen Mary Hospital in Hong Kong from August 1, 2016, to September 31, 2021. Patients were followed up for 2 years. Plasma samples were collected at different longitudinal time points for a prospective circulating tumor DNA (ctDNA) next-generation sequencing profiling study of 77 actionable genes.
Intervention: Patients were treated with up-front surgery, neoadjuvant chemoradiotherapy plus surgery with or without adjuvant therapy, or palliative chemotherapy (CT).
Main outcomes and measures: Detection of circulating tumor DNA (ctDNA), progression-free survival (PFS), and overall survival (OS).
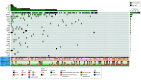
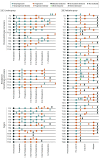
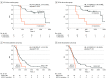
Results: A total of 478 serial plasma samples from 147 patients with locoregional or metastatic ESCC were prospectively analyzed. Among the 74 patients in the curative group (median [range] age, 66 [46-85] years; 56 [76.0%] male), 44 (59.5%) relapsed and 36 (48.6%) died. For patients receiving curative surgical treatment, a high ctDNA level (hazard ratio [HR], 7.84; 95% CI, 1.87-32.97; P = .005) and ctDNA alterations (HR, 5.71; 95% CI, 1.81-17.97; P = .003) at 6 months postoperation were independently associated with poor OS. Among patients receiving neoadjuvant chemoradiotherapy, postneoadjuvant ctDNA alterations were associated with poor PFS (HR, 3.16; 95% CI, 1.17-8.52; P = .02). In the 73 patients in the palliative group (median [range] age, 63 [45-82] years; 63 [86.0%] male), 71 (97.3%) had disease relapse and 68 (93.2%) died. Detectable pre-CT NFE2L2 alterations were independently associated with PFS (HR, 2.99; 95% CI, 1.35-6.61; P = .007) and OS (HR, 28.39; 95% CI, 7.26-111.03; P = 1.52 × 10-6), whereas high ctDNA levels (HR, 2.41; 95% CI, 1.18-4.95; P = .02) and alterations in pre-cycle III ctDNA (HR, 1.99; 95% CI, 1.03-3.85; P = .04) showed weaker associations with PFS. Alterations in pre-CT ctDNA were independently associated with OS (HR, 4.46; 95% CI, 1.86-10.69; P = 7.97 × 10-4).
Conclusions and relevance: The findings of this cohort study indicate that prognostic models incorporating ctDNA features are useful in ESCC. Both ctDNA level and NFE2L2 alterations pre-CT and before cycle III were found to be important prognostic factors in palliative groups, and ctDNA alterations after treatment and at 6 months after surgery may define high-risk groups for recurrence in the curative group. High-risk patients can benefit by a timely switch to the next therapeutic options.
Conflict of interest statement
Figures

Comment in
-
Circulating DNA in Esophageal Cancer-Utility Beyond the Prognostic Application.JAMA Surg. 2023 Nov 1;158(11):1150-1151. doi: 10.1001/jamasurg.2023.4405. JAMA Surg. 2023. PMID: 37728891 No abstract available.
References
-
- Wong C, Law S. Hong Kong experiences of the treatment of esophageal squamous cell carcinoma. In: Ando N, ed. Esophageal Squamous Cell Carcinoma: Diagnosis and Treatment. 2nd ed. Springer Nature; 2020:309-334. doi: 10.1007/978-981-15-4190-2_17 - DOI
-
- Scheuemann P, Hosch SB, Izbicki JR. Prognostic Value of Minimal Residual Disease in Esophageal Cancer. Micrometastasis; 2003:127-138.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

