Repurposing the β3-Adrenergic Receptor Agonist Mirabegron in Patients With Structural Cardiac Disease: The Beta3-LVH Phase 2b Randomized Clinical Trial
- PMID: 37728907
- PMCID: PMC10512168
- DOI: 10.1001/jamacardio.2023.3003
Repurposing the β3-Adrenergic Receptor Agonist Mirabegron in Patients With Structural Cardiac Disease: The Beta3-LVH Phase 2b Randomized Clinical Trial
Abstract
Importance: Left ventricular (LV) hypertrophy contributes to the onset and progression of heart failure (HF), particularly for patients with pre-HF (stage B) for whom no treatment has yet proven effective to prevent transition to overt HF (stage C). The β3-adrenergic receptors (β3ARs) may represent a new target, as their activation attenuates LV remodeling.
Objective: To determine whether activation of β3ARs by repurposing a β3AR agonist, mirabegron, is safe and effective in preventing progression of LV hypertrophy and diastolic dysfunction among patients with pre- or mild HF.
Design, setting, and participants: The Beta3-LVH prospective, triple-blind, placebo-controlled phase 2b randomized clinical trial enrolled patients between September 12, 2016, and February 26, 2021, with a follow-up of 12 months. The trial was conducted at 10 academic hospitals in 8 countries across Europe (Germany, Poland, France, Belgium, Italy, Portugal, Greece, and the UK). Patients aged 18 years or older with or without HF symptoms (maximum New York Heart Association class II) were screened for the presence of LV hypertrophy (increased LV mass index [LVMI] of ≥95 g/m2 for women or ≥115 g/m2 for men) or maximum wall thickness of 13 mm or greater using echocardiography. Data analysis was performed in August 2022.
Intervention: Participants were randomly assigned (1:1) to mirabegron (50 mg/d) or placebo, stratified by the presence of atrial fibrillation and/or type 2 diabetes, for 12 months.
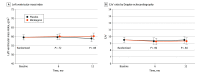
Main outcomes and measures: The primary end points were LVMI determined using cardiac magnetic resonance imaging and LV diastolic function (early diastolic tissue Doppler velocity [E/e'] ratio assessed using Doppler echocardiography) at 12 months. Patients with at least 1 valid measurement of either primary end point were included in the primary analysis. Safety was assessed for all patients who received at least 1 dose of study medication.
Results: Of the 380 patients screened, 296 were enrolled in the trial. There were 147 patients randomized to mirabegron (116 men [79%]; mean [SD] age, 64.0 [10.2] years) and 149 to placebo (112 men [75%]; mean [SD] age, 62.2 [10.9] years). All patients were included in the primary intention-to-treat analysis. At 12 months, the baseline and covariate-adjusted differences between groups included a 1.3-g/m2 increase in LVMI (95% CI, -0.15 to 2.74; P = .08) and a -0.15 decrease in E/e' (95% CI, -0.69 to 0.4; P = .60). A total of 213 adverse events (AEs) occurred in 82 mirabegron-treated patients (including 31 serious AEs in 19 patients) and 215 AEs occurred in 88 placebo-treated patients (including 30 serious AEs in 22 patients). No deaths occurred during the trial.
Conclusions: In this study, mirabegron therapy had a neutral effect on LV mass or diastolic function over 12 months among patients who had structural heart disease with no or mild HF symptoms.
Trial registration: ClinicalTrials.gov Identifier: NCT02599480.
Conflict of interest statement
Figures

References
-
- Heidenreich PA, Bozkurt B, Aguilar D, et al. . 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876-e894. doi:10.1161/CIR.0000000000001062 - DOI - PubMed
-
- Bozkurt B, Coats AJS, Tsutsui H, et al. . Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23(3):352-380. doi:10.1002/ejhf.2115 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

