Exploring the "misfolding problem" by systematic discovery and analysis of functional-but-degraded proteins
- PMID: 37729018
- PMCID: PMC10848938
- DOI: 10.1091/mbc.E23-06-0248
Exploring the "misfolding problem" by systematic discovery and analysis of functional-but-degraded proteins
Abstract
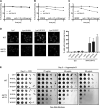
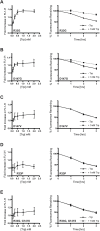
In both health and disease, the ubiquitin-proteasome system (UPS) degrades point mutants that retain partial function but have decreased stability compared with their wild-type counterparts. This class of UPS substrate includes routine translational errors and numerous human disease alleles, such as the most common cause of cystic fibrosis, ΔF508-CFTR. Yet, there is no systematic way to discover novel examples of these "minimally misfolded" substrates. To address that shortcoming, we designed a genetic screen to isolate functional-but-degraded point mutants, and we used the screen to study soluble, monomeric proteins with known structures. These simple parent proteins yielded diverse substrates, allowing us to investigate the structural features, cytotoxicity, and small-molecule regulation of minimal misfolding. Our screen can support numerous lines of inquiry, and it provides broad access to a class of poorly understood but biomedically critical quality-control substrates.
Figures





Similar articles
-
Cystic fibrosis transmembrane conductance regulator degradation: cross-talk between the ubiquitylation and SUMOylation pathways.FEBS J. 2013 Sep;280(18):4430-8. doi: 10.1111/febs.12415. Epub 2013 Jul 22. FEBS J. 2013. PMID: 23809253 Free PMC article. Review.
-
SYVN1, NEDD8, and FBXO2 Proteins Regulate ΔF508 Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Ubiquitin-mediated Proteasomal Degradation.J Biol Chem. 2016 Dec 2;291(49):25489-25504. doi: 10.1074/jbc.M116.754283. Epub 2016 Oct 18. J Biol Chem. 2016. PMID: 27756846 Free PMC article.
-
Human heat shock protein 105/110 kDa (Hsp105/110) regulates biogenesis and quality control of misfolded cystic fibrosis transmembrane conductance regulator at multiple levels.J Biol Chem. 2012 Jun 1;287(23):19158-70. doi: 10.1074/jbc.M111.297580. Epub 2012 Apr 13. J Biol Chem. 2012. PMID: 22505710 Free PMC article.
-
Selective inhibition of endoplasmic reticulum-associated degradation rescues DeltaF508-cystic fibrosis transmembrane regulator and suppresses interleukin-8 levels: therapeutic implications.J Biol Chem. 2006 Jun 23;281(25):17369-17378. doi: 10.1074/jbc.M600509200. Epub 2006 Apr 18. J Biol Chem. 2006. PMID: 16621797
-
The role of the UPS in cystic fibrosis.BMC Biochem. 2007 Nov 22;8 Suppl 1(Suppl 1):S11. doi: 10.1186/1471-2091-8-S1-S11. BMC Biochem. 2007. PMID: 18047735 Free PMC article. Review.
Cited by
-
The kinesin Kar3 is required for endoplasmic reticulum-associated degradation.Mol Biol Cell. 2025 Mar 1;36(3):br9. doi: 10.1091/mbc.E24-10-0437. Epub 2025 Jan 22. Mol Biol Cell. 2025. PMID: 39841550 Free PMC article.
-
Endoplasmic reticulum and inner nuclear membrane ubiquitin-conjugating enzymes Ubc6 and Ubc7 confer resistance to hygromycin B in Saccharomyces cerevisiae.MicroPubl Biol. 2024 Jul 29;2024:10.17912/micropub.biology.001276. doi: 10.17912/micropub.biology.001276. eCollection 2024. MicroPubl Biol. 2024. PMID: 39139584 Free PMC article.
-
Structural basis for anomalous cellular trafficking behavior of glaucoma-associated A427T mutant myocilin.bioRxiv [Preprint]. 2025 Feb 27:2025.02.26.640437. doi: 10.1101/2025.02.26.640437. bioRxiv. 2025. PMID: 40060664 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

