Mechanisms of action of incretin receptor based dual- and tri-agonists in pancreatic islets
- PMID: 37729025
- PMCID: PMC10874655
- DOI: 10.1152/ajpendo.00236.2023
Mechanisms of action of incretin receptor based dual- and tri-agonists in pancreatic islets
Abstract
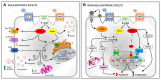
Simultaneous activation of the incretin G-protein-coupled receptors (GPCRs) via unimolecular dual-receptor agonists (UDRA) has emerged as a new therapeutic approach for type 2 diabetes. Recent studies also advocate triple agonism with molecules also capable of binding the glucagon receptor. In this scoping review, we discuss the cellular mechanisms of action (MOA) underlying the actions of these novel and therapeutically important classes of peptide receptor agonists. Clinical efficacy studies of several UDRAs have demonstrated favorable results both as monotherapies and when combined with approved hypoglycemics. Although the additive insulinotropic effects of dual glucagon-like peptide-1 receptor (GLP-1R) and glucose-dependent insulinotropic peptide receptor (GIPR) agonism were anticipated based on the known actions of either glucagon-like peptide-1 (GLP-1) or glucose-dependent insulinotropic peptide (GIP) alone, the additional benefits from GCGR were largely unexpected. Whether additional synergistic or antagonistic interactions among these G-protein receptor signaling pathways arise from simultaneous stimulation is not known. The signaling pathways affected by dual- and tri-agonism require more trenchant investigation before a comprehensive understanding of the cellular MOA. This knowledge will be essential for understanding the chronic efficacy and safety of these treatments.
Keywords: glucagon; glucagon-like peptide 1; glucose-dependent insulinotropic peptide; islets of Langerhans; type 2 diabetes mellitus.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Labarre J. Sur les possibilities d’un traitement du diabete par. L’incretine. Bull Acad R Med Belg 12: 620–634, 1932.

