Shedding light on rechargeable Na/Cl2 battery
- PMID: 37729201
- PMCID: PMC10523539
- DOI: 10.1073/pnas.2310903120
Shedding light on rechargeable Na/Cl2 battery
Abstract
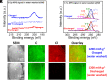
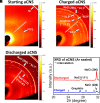
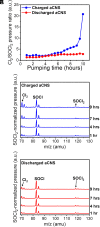
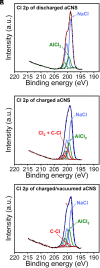
Advancing new ideas of rechargeable batteries represents an important path to meeting the ever-increasing energy storage needs. Recently, we showed rechargeable sodium/chlorine (Na/Cl2) (or lithium/chlorine Li/Cl2) batteries that used a Na (or Li) metal negative electrode, a microporous amorphous carbon nanosphere (aCNS) positive electrode, and an electrolyte containing dissolved aluminum chloride and fluoride additives in thionyl chloride [G. Zhu et al., Nature 596, 525-530 (2021) and G. Zhu et al., J. Am. Chem. Soc. 144, 22505-22513 (2022)]. The main battery redox reaction involved conversion between NaCl and Cl2 trapped in the carbon positive electrode, delivering a cyclable capacity of up to 1,200 mAh g-1 (based on positive electrode mass) at a ~3.5 V discharge voltage [G. Zhu et al., Nature 596, 525-530 (2021) and G. Zhu et al., J. Am. Chem. Soc. 144, 22505-22513 (2022)]. Here, we identified by X-ray photoelectron spectroscopy (XPS) that upon charging a Na/Cl2 battery, chlorination of carbon in the positive electrode occurred to form carbon-chlorine (C-Cl) accompanied by molecular Cl2 infiltrating the porous aCNS, consistent with Cl2 probed by mass spectrometry. Synchrotron X-ray diffraction observed the development of graphitic ordering in the initially amorphous aCNS under battery charging when the carbon matrix was oxidized/chlorinated and infiltrated with Cl2. The C-Cl, Cl2 species and graphitic ordering were reversible upon discharge, accompanied by NaCl formation. The results revealed redox conversion between NaCl and Cl2, reversible graphitic ordering/amorphourization of carbon through battery charge/discharge, and probed trapped Cl2 in porous carbon by XPS.
Keywords: battery; chemistry; energy storage; material sciences.
Conflict of interest statement
One of the reviewers, H.W., coauthored an award announcement with the corresponding author H.D. in 2019. The two groups have not had any collaborations in the past 10 y.
Figures

References
-
- Feng Y., et al. , Challenges and advances in wide-temperature rechargeable lithium batteries. Energy Environ. Sci. 15, 1711–1759 (2022).
-
- Goodenough J. B., Park K.-S., The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013). - PubMed
-
- Cohn A. P., Muralidharan N., Carter R., Share K., Pint C. L., Anode-free sodium battery through in situ plating of sodium metal. Nano Lett. 17, 1296–1301 (2017). - PubMed
-
- Liang P., et al. , A nonflammable high-voltage 4.7 V anode-free lithium battery. Adv. Mater. 34, e2207361 (2022). - PubMed
LinkOut - more resources
Full Text Sources

