Synthesis of N-alkoxycarbonyl Pyrroles from O-Substituted Carbamates: A Synthetically Enabling Pyrrole Protection Strategy
- PMID: 37729493
- PMCID: PMC10563134
- DOI: 10.1021/acs.joc.3c01257
Synthesis of N-alkoxycarbonyl Pyrroles from O-Substituted Carbamates: A Synthetically Enabling Pyrrole Protection Strategy
Abstract
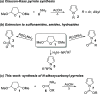
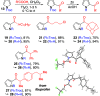
The condensation of readily available O-substituted carbamates with 2,5-dimethoxytetrahydrofuran gives N-alkoxycarbonyl pyrroles in a single step and in good yield. By this method, several common amine protecting groups can be introduced on the pyrrole nitrogen. With the exception of N-Boc, N-alkoxycarbonyl groups have seen only minimal use for protection of the pyrrole nitrogen to date. Here, we show that N-alkoxycarbonyl protection can endow pyrrole with distinct reactivity in comparison with N-sulfonyl protection, for example, in a pyrrole acylation protocol employing carboxylic acids with a sulfonic acid anhydride activator.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


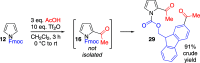

Similar articles
-
Protecting Group-Dependent Synthesis of Densely Substituted Dihydropyrroles v/s Pyrroles via 5-Exo-trig Cascade Radical Cyclization to Alkynyl Vinylogous Carbamates.J Org Chem. 2022 May 20;87(10):6781-6793. doi: 10.1021/acs.joc.2c00473. Epub 2022 May 11. J Org Chem. 2022. PMID: 35544612
-
Clauson-Kaas pyrrole synthesis using diverse catalysts: a transition from conventional to greener approach.Beilstein J Org Chem. 2023 Jun 27;19:928-955. doi: 10.3762/bjoc.19.71. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37404802 Free PMC article. Review.
-
Aryl-Extended and Super Aryl-Extended Calix[4]pyrroles: Design, Synthesis, and Applications.Acc Chem Res. 2023 Feb 21;56(4):500-513. doi: 10.1021/acs.accounts.2c00839. Epub 2023 Feb 2. Acc Chem Res. 2023. PMID: 36734050
-
Highly regioselective synthesis of 3,4-disubstituted 1H-pyrrole.J Org Chem. 2000 Jun 2;65(11):3274-83. doi: 10.1021/jo991531c. J Org Chem. 2000. PMID: 10843606
-
[New reactivities of nitrogen-containing aromatics and their synthetic application].Yakugaku Zasshi. 2002 Nov;122(11):869-88. doi: 10.1248/yakushi.122.869. Yakugaku Zasshi. 2002. PMID: 12440147 Review. Japanese.
References
-
- Singh N.; Singh S.; Kohli S.; Singh A.; Asiki H.; Rathee G.; Chandra R.; Anderson E. A. Recent Progress in the Total Synthesis of Pyrrole-Containing Natural Products. Org. Chem. Front. 2021, 8, 5550.10.1039/D0QO01574A. - DOI
-
- Bhardwaj V.; Gumber D.; Abbot V.; Dhiman S.; Sharma P. Pyrrole: A Resourceful Small Molecule in Key Medicinal Hetero-Aromatics. RSC Adv. 2015, 5, 15233.10.1039/C4RA15710A. - DOI
LinkOut - more resources
Full Text Sources

