One-Pot Synthesis of Sulfonamides from Unactivated Acids and Amines via Aromatic Decarboxylative Halosulfonylation
- PMID: 37729614
- PMCID: PMC10680120
- DOI: 10.1021/jacs.3c08218
One-Pot Synthesis of Sulfonamides from Unactivated Acids and Amines via Aromatic Decarboxylative Halosulfonylation
Abstract
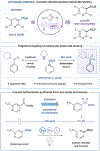
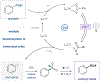
The coupling of carboxylic acids and amines to form amide linkages is the most commonly performed reaction in the pharmaceutical industry. Herein, we report a new strategy that merges these traditional amide coupling partners to generate sulfonamides, important amide bioisosteres. This method leverages copper ligand-to-metal charge transfer (LMCT) to convert aromatic acids to sulfonyl chlorides, followed by one-pot amination to form the corresponding sulfonamide. This process requires no prefunctionalization of the native acid or amine and extends to a diverse set of aryl, heteroaryl, and s-rich aliphatic substrates. Further, we extend this strategy to the synthesis of (hetero)aryl sulfonyl fluorides, which have found utility as "click" handles in chemical probes and programmable bifunctional reagents. Finally, we demonstrate the utility of these protocols in pharmaceutical analogue synthesis.
Conflict of interest statement
The authors declare the following competing financial interest(s): D.W.C.M. declares a competing financial interest with respect to the Integrated Photoreactor.
Figures



References
-
- Varenikov A; Shapiro E; Gandelman M Decarboxylative Halogenation of Organic Compounds. Chem. Rev 2021, 121 (1), 412–484. - PMC - PubMed
- Beil SB; Chen TQ; Intermaggio NE; MacMillan DWC Carboxylic Acids as Adaptive Functional Groups in Metallaphotoredox Catalysis. Acc. Chem. Res 2022, 55 (23), 3481–3494. - PMC - PubMed
-
- Wang Y; Haight I; Gupta R; Vasudevan A What Is in Our Kit? An Analysis of Building Blocks Used in Medicinal Chemistry Parallel Libraries. J. Med. Chem 2021, 64 (23), 17115–17122. - PubMed
-
- Boström J; Brown DG; Young RJ; Keserü GM Expanding the Medicinal Chemistry Synthetic Toolbox. Nat. Rev. Drug Discovery 2018, 17 (10), 709–727. - PubMed
-
- For examples of alternative couplings of carboxylic acids and amines, see:
- Zhang Z; Cernak T The Formal Cross-Coupling of Amines and Carboxylic Acids to Form sp3−sp3 Carbon−Carbon Bonds. Angew. Chem., Int. Ed 2021, 60 (52), 27293–27298. - PubMed
- McGrath A; Zhang R; Shafiq K; Cernak T Repurposing amine and carboxylic acid building blocks with an automatable esterification reaction. Chem. Commun 2023, 59, 1026–1029. - PubMed
- Wang J; Ehehalt LE; Huang Z; Beleh OM; Guzei IA; Weix DJ Formation of C(sp2)−C(sp3) Bonds Instead of Amide C−N Bonds from Carboxylic Acid and Amine Substrate Pools by Decarbonylative Cross-Electrophile Coupling. J. Am. Chem. Soc 2023, 145 (18), 9951–9958. - PMC - PubMed
- Douthwaite JL; Zhao R; Shim E; Mahjour B; Zimmerman PM; Cernak T Formal Cross-Coupling of Amines and Carboxylic Acids to Form sp3−sp2 Carbon−Carbon Bonds. J. Am. Chem. Soc 2023, 145 (20), 10930–10937. - PMC - PubMed
-
- Mahjour B; Shen Y; Liu W; Cernak T A map of the amine–carboxylic acid coupling system. Nature. 2020, 580, 71–75. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

