Adenosine A2A receptor is a tumor suppressor of NASH-associated hepatocellular carcinoma
- PMID: 37729873
- PMCID: PMC10518627
- DOI: 10.1016/j.xcrm.2023.101188
Adenosine A2A receptor is a tumor suppressor of NASH-associated hepatocellular carcinoma
Abstract
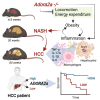
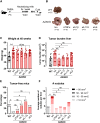
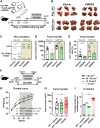
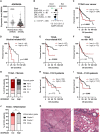
Inhibition of adenosine A2A receptor (A2AR) is a promising approach for cancer immunotherapy currently evaluated in several clinical trials. We here report that anti-obesogenic and anti-inflammatory functions of A2AR, however, significantly restrain hepatocellular carcinoma (HCC) development. Adora2a deletion in mice triggers obesity, non-alcoholic steatohepatitis (NASH), and systemic inflammation, leading to spontaneous HCC and promoting dimethylbenzyl-anthracene (DMBA)- or diethylnitrosamine (DEN)-induced HCC. Conditional Adora2a deletion reveals critical roles of myeloid and hepatocyte-derived A2AR signaling in restraining HCC by limiting hepatic inflammation and steatosis. Remarkably, the impact of A2AR pharmacological blockade on HCC development is dependent on pre-existing NASH. In support of our animal studies, low ADORA2A gene expression in human HCC is associated with cirrhosis, hepatic inflammation, and poor survival. Together, our study uncovers a previously unappreciated tumor-suppressive function for A2AR in the liver and suggests caution in the use of A2AR antagonists in patients with NASH and NASH-associated HCC.
Keywords: A2A receptor; HCC; NAFLD; NASH; adenosine; cancer; immuno-oncology; inflammation.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.S. is a paid consultant, SAB member, and owns stocks in Surface Oncology and received sponsored research grants from Surface Oncology.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

