Olfactory bulb activity shapes the development of entorhinal-hippocampal coupling and associated cognitive abilities
- PMID: 37729915
- PMCID: PMC10617757
- DOI: 10.1016/j.cub.2023.08.072
Olfactory bulb activity shapes the development of entorhinal-hippocampal coupling and associated cognitive abilities
Abstract
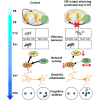
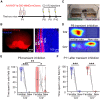
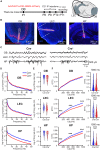
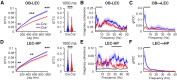
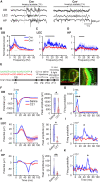
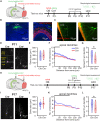
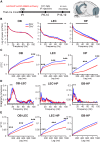
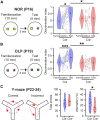
The interplay between olfaction and higher cognitive processing has been documented in the adult brain; however, its development is poorly understood. In mice, shortly after birth, endogenous and stimulus-evoked activity in the olfactory bulb (OB) boosts the oscillatory entrainment of downstream lateral entorhinal cortex (LEC) and hippocampus (HP). However, it is unclear whether early OB activity has a long-lasting impact on entorhinal-hippocampal function and cognitive processing. Here, we chemogenetically silenced the synaptic outputs of mitral/tufted cells, the main projection neurons in the OB, during postnatal days 8-10. The transient manipulation leads to a long-lasting reduction of oscillatory coupling and weaker responsiveness to stimuli within developing entorhinal-hippocampal circuits accompanied by dendritic sparsification of LEC pyramidal neurons. Moreover, the transient silencing reduces the performance in behavioral tests involving entorhinal-hippocampal circuits later in life. Thus, neonatal OB activity is critical for the functional LEC-HP development and maturation of cognitive abilities.
Keywords: chemogenetics; development; entorhinal-hippocampal network; olfactory bulb; oscillatory activity; recognition memory.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
Comment in
-
Neuroscience: Building better cognition through smell.Curr Biol. 2023 Oct 23;33(20):R1049-R1051. doi: 10.1016/j.cub.2023.09.030. Curr Biol. 2023. PMID: 37875078
Similar articles
-
Olfactory-driven beta band entrainment of limbic circuitry during neonatal development.J Physiol. 2023 Aug;601(16):3605-3630. doi: 10.1113/JP284401. Epub 2023 Jul 11. J Physiol. 2023. PMID: 37434507
-
Coordinated electrical activity in the olfactory bulb gates the oscillatory entrainment of entorhinal networks in neonatal mice.PLoS Biol. 2019 Jan 31;17(1):e2006994. doi: 10.1371/journal.pbio.2006994. eCollection 2019 Jan. PLoS Biol. 2019. PMID: 30703080 Free PMC article.
-
Bursting mitral cells time the oscillatory coupling between olfactory bulb and entorhinal networks in neonatal mice.J Physiol. 2020 Dec;598(24):5753-5769. doi: 10.1113/JP280131. Epub 2020 Oct 4. J Physiol. 2020. PMID: 32926437
-
Circuit dynamics of the olfactory pathway during olfactory learning.Front Neural Circuits. 2024 Jul 5;18:1437575. doi: 10.3389/fncir.2024.1437575. eCollection 2024. Front Neural Circuits. 2024. PMID: 39036422 Free PMC article. Review.
-
Processing of cell assemblies in the lateral entorhinal cortex.Rev Neurosci. 2022 Apr 22;33(8):829-847. doi: 10.1515/revneuro-2022-0011. Print 2022 Dec 16. Rev Neurosci. 2022. PMID: 35447022 Review.
Cited by
-
Modifying Alzheimer's disease pathophysiology with photobiomodulation: model, evidence, and future with EEG-guided intervention.Front Neurol. 2024 Aug 23;15:1407785. doi: 10.3389/fneur.2024.1407785. eCollection 2024. Front Neurol. 2024. PMID: 39246604 Free PMC article. Review.
-
The potential of olfaction loss to induce cognitive impairment and anxiety behavior in mice via the microbiota-gut-brain axis.Front Microbiol. 2025 Jul 2;16:1595742. doi: 10.3389/fmicb.2025.1595742. eCollection 2025. Front Microbiol. 2025. PMID: 40673140 Free PMC article.
-
Olfactory epithelium electrical stimulation mitigates memory and synaptic deficits caused by mechanical ventilation.Sci Rep. 2025 Apr 9;15(1):12197. doi: 10.1038/s41598-025-96661-9. Sci Rep. 2025. PMID: 40204831 Free PMC article.
-
Protocol for adeno-associated virus-mediated optogenetic activation of olfactory output neurons in neonatal mice.STAR Protoc. 2024 Sep 20;5(3):103164. doi: 10.1016/j.xpro.2024.103164. Epub 2024 Jul 4. STAR Protoc. 2024. PMID: 38968078 Free PMC article.
-
Bibliometric analysis of hot literature on neural circuit research.Ibrain. 2023 Dec 21;10(1):69-82. doi: 10.1002/ibra.12144. eCollection 2024 Spring. Ibrain. 2023. PMID: 38682019 Free PMC article. Review.
References
-
- Quirk C.R., Zutshi I., Srikanth S., Fu M.L., Devico Marciano N., Wright M.K., Parsey D.F., Liu S., Siretskiy R.E., Huynh T.L., et al. Precisely timed theta oscillations are selectively required during the encoding phase of memory. Nat. Neurosci. 2021;24:1614–1627. doi: 10.1038/s41593-021-00919-0. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

