Clinical Pathway for Coronary Atherosclerosis in Patients Without Conventional Modifiable Risk Factors: JACC State-of-the-Art Review
- PMID: 37730292
- PMCID: PMC10522922
- DOI: 10.1016/j.jacc.2023.06.045
Clinical Pathway for Coronary Atherosclerosis in Patients Without Conventional Modifiable Risk Factors: JACC State-of-the-Art Review
Abstract
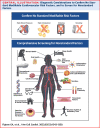
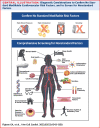
Reducing the incidence and prevalence of standard modifiable cardiovascular risk factors (SMuRFs) is critical to tackling the global burden of coronary artery disease (CAD). However, a substantial number of individuals develop coronary atherosclerosis despite no SMuRFs. SMuRFless patients presenting with myocardial infarction have been observed to have an unexpected higher early mortality compared to their counterparts with at least 1 SMuRF. Evidence for optimal management of these patients is lacking. We assembled an international, multidisciplinary team to develop an evidence-based clinical pathway for SMuRFless CAD patients. A modified Delphi method was applied. The resulting pathway confirms underlying atherosclerosis and true SMuRFless status, ensures evidence-based secondary prevention, and considers additional tests and interventions for less typical contributors. This dedicated pathway for a previously overlooked CAD population, with an accompanying registry, aims to improve outcomes through enhanced adherence to evidence-based secondary prevention and additional diagnosis of modifiable risk factors observed.
Keywords: atherosclerosis; cardiovascular risk; clinical pathway; coronary artery disease; primary prevention; secondary prevention.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures The Centre for Research Excellence is supported by National Health & Medical Research Council of Australia (grant number GNT1196629). Dr Figtree is funded by a National Health & Medical Research Council Practitioner Fellowship (GNT1135920). Dr Ellinor is supported by grants from the National Institutes of Health (1RO1HL092577, 1R01HL157635, 1R01HL157635), by a grant from the American Heart Association Strategically Focused Research Networks (18SFRN34110082), and by a grant from the European Union (MAESTRIA 965286). Dr Psaltis is funded by a National Heart Foundation Level 3 Future Leader Fellowship (106656). Dr Redfern is supported by grants from the National Health & Medical Research Council of Australia (GNT2007946 and GNT1182301). Dr Wilson is supported by grants from the National Health & Medical Research Council of Australia (GNT119600, GNT9100001, GNT1153479). Dr Guzik is funded by the British Heart Foundation and European Research Council (ERC-InflammaTENSION 726318). Dr Arnott has received honoraria from Amgen and AstraZeneca. Dr Brown has received consulting fees from Novartis. Dr Bhatt has received research funding from Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, Cincor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Pharmaceuticals, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer Inc, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89bio; has received royalties from Elsevier; has received consulting fees from Broadview Ventures, Hims, and McKinsey; has received payment or honoraria from the American College of Cardiology, Baim Institute for Clinical Research, Belvoir Publications, Boston Scientific, Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Novartis, Population Health Research Institute, Rutgers University, Canadian Medical and Surgical Knowledge Translation Research Group, Cowen and Company, HMP Global, Journal of the American College of Cardiology, K2P, Level Ex, Medtelligence/ReachMD, MJH Life Sciences, Oakstone CME, Piper Sandler, Slack Publications, WebMD, Wiley, and Society of Cardiovascular Patient Care; has received payment for expert testimony from Arnold and Porter law firm; has received travel or meeting support from the American College of Cardiology, Society of Cardiovascular Patient Care, and American Heart Association; has been named (no income) on a patent application for sotagliflozin, assigned to Brigham and Women’s Hospital, who assigned to Lexicon; serves on a Data Safety Monitoring Board for Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute, Boston Scientific, Cleveland Clinic, Contego Medical, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Novartis, and Population Health Research Institute; has served on an Advisory Board for AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; has served on the Board of Directors for the American College of Cardiology, AngioWave, Boston VA Research Institute, Bristol Myers Squibb, DRS.LINQ, High Enroll, Society of Cardiovascular Patient Care, and TobeSoft; has stock or stock options with AngioWave, Bristol Myers Squibb, DRS.LINQ, and High Enroll; has financial or other nonfinancial interests in Clinical Cardiology, NCDR-ACTION Registry Steering Committee, Contego Medical, American Heart Association Quality Oversight Committee, VA CART Research and Publications Committee; and is a coinvestigator for Abbott, Biotronik, Boston Scientific, CSI, St Jude Medical, Phillips SpectraWAVE, Svelte, and Vascular Solutions. Dr Brieger has received research grant support from Novartis and has received honoraria from BMS/Pfizer. Dr Chong has received consulting fees from Implicit Bioscience Pty; and has a provisional patent for a cell surface marker signature for arrhythmogenic pluripotent stem cell-derived cardiomyocyte. Dr Cistulli has received research grant support from ResMed and SomnoMed; has received consulting fees from RedMed, SomnoMed, and Signifier Medical Technologies; and has received honoraria from ResMed and SomnoMed. Dr Ellinor has received sponsored research support from Bayer AG and IBM Research; and has served on Advisory Boards or consulted for Bayer AG, MyoKardia, and Novartis. Dr Figtree has received personal fees from Amgen, AstraZeneca, Bayer, CSL, and Janssen; has received grants from Abbott Diagnostic and Sanofi; is a founding director and chief medical officer of Prokardia; has a patent, “Biomarkers and Oxidative Stress,” awarded in the United States in May 2017 (US9638699B2) licensed to Northern Sydney Local Health District; has a patent, “Use of P2X7R Antagonists in Cardiovascular Disease” (PCT/AU2018/050905), licensed to Prokardia; has a patent, “Methods for Treatment and Prevention of Vascular Disease” (PCT/AU2015/000548) licensed to the University of Sydney/Northern Sydney Local Health District; and has a filed provisional patent application, “Methods for Predicting Coronary Artery Disease” (USYD Ref: 2022-009-PRO-0; 2022902660) to the University of Sydney/Northern Sydney Local Health District. Dr Hagström has received research grant support to his institution from Amgen and Pfizer; and has received honoraria from Amgen, AstraZeneca, Bayer, Novo Nordisk, Amarin, and Sanofi. Dr Jenkins has received research grant support from Abbott, Medtronic, and Mylan; and has served on Advisory Boards for Abbott Diabetes Care, Amgen, Insulet, and Medtronic. Dr Keech has received grant support from Abbott, Amgen, and Mylan; has received consulting fees from AstraZeneca, Pfizer, and Sanofi; and has participated as a Data Safety Monitoring Board member for the PROMINENT trial (Kowa Research Institute). Dr Kritharides has received research grant support from Amgen; and has received consulting fees from Seqiris. Dr Meikle has a license agreement with Juvenescence Ltd; and has received consultancy payments (made to Baker Institute) from BCAL Scientific and Juvenescence Ltd. Dr Mehran has received research grant support to her institution from Abbott, Abiomed, Alleviant Medical, AM-Pharma, Amgen, Applied Therapeutics, Arena, AstraZeneca, AtriCure, BAIM, Bayer, Beth Israel Deaconess Biosensors, Biotronik, Boston Scientific, Bristol Myers Squibb, CardiaWave, CellAegis, CeloNova, CERC, Chiesi, Concept Medical, CSL Behring, Cytosorbents, Daiichi-Sankyo, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe AG, Magenta, Medtronic, Novartis, OrbusNeich, PhaseBio, Philips, RenalPro, RM Global, Shockwave, and Vivasure Zoll; has received consulting fees from Cine-Med Research; has served on an Advisory Board with all payments made to institution for Abbott, Janssen, Medtronic, and Novartis; and she and/or her spouse has stock or stock options in Applied Therapeutics, ControlRad, Elixir Medical, and Stel. Dr Natarajan has received research grant support from Apple, Amgen, AstraZeneca, Boston Scientific, and Novartis; has received consulting fees from Apple, AstraZeneca, Blackstone Life Sciences, Foresite Labs, Genetech/Roche, Invitae, Novartis, and TenSixteen Bio; has served on Advisory Boards for Esperion Therapeutics, geneXwell, and TenSixteen Bio; and holds stock or stock options in TenSixteen Bio and geneXwell. Dr Nicholls has received research grant support from AstraZeneca, Amgen, Anthera, Cerenis, Eli Lilly, Esperion, InfraReDx, LipoScience, The Medicines Company, New Amsterdam Pharma, Novartis, Resverlogix, Roche, and Sanofi-Regeneron; and has received consulting fees from Akcea, Amarin, Anthera, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Omthera, Merck, Resverlogix, Sanofi-Regeneron, Takeda, and Vaxxinity. Dr Psaltis has received consulting fees from Amgen, Esperion Therapeutics, and Novartis; has received speaker honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, and Sanofi; has received meeting travel support from Amgen and AstraZeneca; and has submitted a provisional patent for a method and composition for promoting neovascularization. Dr Sundström has stock or stock options with Anagram Kommunikation AB and Symptoms Europe AB. Dr Steg has received research grant support from Amarin, Bayer, Sanofi, and Servier Laboratories; has received consulting fees from Amgen, AstraZeneca, BMS/Myokardia, Merck, Novo Nordisk, and Regeneron; has served on Steering Committees or Critical Event Committees for Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Idorsia, Novartis, PhaseBio, Pfizer, Sanofi, and Servier; has received honoraria from AstraZeneca, Novartis, and Novo Nordisk; and has served on Data Safety Monitoring Boards for Servier, Sanofi, and PHRI. Dr Guzik has received research funding from the European Commission (ImmuneHyperCog and BrainGutCVD studies); has served as Editor-in-Chief for Cardiovascular Research; and has served as Board Committee member for the European Society of Cardiology. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Mendis S., Puska P., Norrving B., World Health Organization, World Heart Federation, World Stroke Organization . World Health Organization; 2011. Global Atlas on Cardiovascular Disease Prevention and Control.
-
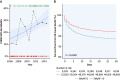
- Vernon S.T., Coffey S., Bhindi R., et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur J Prev Cardiol. 2017;24:1824–1830. - PubMed
-
- Kong G., Chin Y.H., Chong B., et al. Higher mortality in acute coronary syndrome patients without standard modifiable risk factors: results from a global meta-analysis of 1,285,722 patients. Int J Cardiol. 2023;371:432–440. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

