Efficacy of transcutaneous auricular vagus nerve stimulation on radiotherapy-related neuropathic pain in patients with head and neck cancers (RELAX): protocol for a multicentre, randomised, double-blind, sham-controlled trial
- PMID: 37730386
- PMCID: PMC10514600
- DOI: 10.1136/bmjopen-2023-072724
Efficacy of transcutaneous auricular vagus nerve stimulation on radiotherapy-related neuropathic pain in patients with head and neck cancers (RELAX): protocol for a multicentre, randomised, double-blind, sham-controlled trial
Abstract
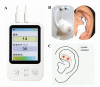
Introduction: Radiotherapy-related neuropathic pain (RRNP) is one of the most distressing complications after radiotherapy for head and neck cancers. Drug therapy is not sufficiently effective and has limitations in terms of dose titration period and side effects. Transcutaneous auricular vagus nerve stimulation (taVNS), which stimulates the auricular branches of the vagus nerve through electrical impulses, has been proven to have analgesic effects in certain diseases. However, it is unknown whether taVNS can relieve RRNP.
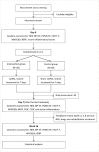
Methods and analysis: This is a multicentre, randomised, double-blind, parallel, sham-controlled trial. We will include adult patients newly diagnosed with neuropathic pain after radiotherapy for head and neck cancers. One hundred and sixteen individuals will be recruited and randomly assigned in a 1:1 ratio to receive taVNS or sham stimulation. The interventions will last for 7 days, twice daily for 30 min each. The primary efficacy outcome is pain reduction on day 7. The secondary outcomes are changes in functional interference, psychological distress, fatigue, quality of life and serum inflammatory factors. The study may provide a new early intervention strategy for RRNP among patients with head and neck cancers.
Ethics and dissemination: This study has been approved by the Medical Research Ethics Committee of Sun Yat-sen University (SYSKY-2022-109-01) and will be conducted in strict accordance with the Declaration of Helsinki. Ethical approvals will be obtained separately for all centres involved in the study. Study results will be published in peer-reviewed academic journals. The database of the study will be available from the corresponding author on reasonable request.
Trial registration number: NCT05543239.
Keywords: Head & neck tumours; Neurological pain; Pain management.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Leenstra JL, Miller RC, Qin R, et al. . Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: a phase III, randomized, double-blind trial (NCCTG-N09C6 [alliance]). J Clin Oncol 2014;32:1571–7. 10.1200/JCO.2013.53.2630 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
